Abstract
The overall burden of chronic musculoskeletal pain in Asian countries will continue to increase as the population ages, as will the demand for safe and effective pain management. Currently available Asian guidelines are mostly outdated and targeted only to primary care. Implementation of international guidelines may be unsuitable for Asian patients due to cultural, local economic and regulatory factors. With the aim of developing Asian-specifi c consensus recommendations for the pharmacological management of osteoarthritis (OA) pain and chronic low back pain (cLBP), we convened to review and discuss recent available evidence for pharmacotherapy, clinical experiences, and current practice challenges they face in the region, including challenges in opioid use. Taking these into consideration, we provided general recommendations for the overall assessment and management of OA pain and cLBP. The strength of the recommendations regarding the use of pharmacological agents was assessed using the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system. Where evidence is confl icting or limited, we made no recommendation pending the availability of further evidence. We recommend topical non-steroidal anti-infl ammatory drugs (NSAIDs) as a fi rst-line pharmacological treatment of OA pain, while oral NSAIDs should be considered as a fi rst-line pharmacological treatment of cLBP. Acetaminophen has been commonly used as the fi rst-line treatment for OA pain and cLBP, but its long-term use is not recommended based on recent evidence. These consensus recommendations are not prescriptive, and serve as a guide for decision-making in clinical practice. The optimal management of OA pain and cLBP should ultimately be individualized to each patient.
Keywords
pharmacotherapy, osteoarthritis pain, low back pain, Asian consensus, musculoskeletal pain
Introduction
The International Association for the Study of Pain Task Force defines chronic musculoskeletal pain as “persistent or recurrent pain that arises as part of a disease process directly affecting bone(s), joint(s), muscle(s), or related soft tissue(s).” This definition is limited to nociceptive (peripheral) pain, and includes pain characterized by persistent inflammation such as rheumatoid arthritis and by structural changes that affect bones, joints, tendons, or muscles such as osteoarthritis (OA).1 Musculoskeletal conditions with causes that are not completely understood, such as nonspecific back pain, are classified under chronic primary pain—defined as “pain in one or more anatomic regions that persists or recurs for longer than 3 months and is associated with significant emotional distress or significant functional disability and that cannot be better explained by another chronic pain condition.”1 Similar to chronic low back pain (cLBP; the abbreviation LBP is used instead when studies do not specify duration of pain), current data seem to support the description of OA as a mixed pain state, where both nociceptive and neuropathic factors may play important roles in some individuals.2
The prevalence of musculoskeletal pain is expected to increase in the Asia region due to population aging, highlighting the need for an evidence-based approach to optimally manage chronic musculoskeletal pain in the Asian population. However, some of the available guidelines in Asia on the management of chronic musculoskeletal pain are outdated and targeted only to primary care.3,4 Some of the existing international guidelines that are commonly referred to in clinical practice are at least 10 years old and the scientific evidence assessed by these guidelines are at least a few years older than the date of publication. Cultural, local economic, and regulatory factors may render the clinical implementation of European or American guidelines unsuitable for Asian patients.5,6 In view of the above factors, the current manuscript aims to provide an updated consensus on Asian-specific recommendations in the pharmacological management of OA pain and cLBP based on the availability of newer evidence.
Methods
A consensus meeting was held in April 2016 in Incheon, South Korea, to address the need for Asian-specific recommendations on both OA and cLBP as both exhibit similar pathology in chronic pain state. Due to limited Asian-specific pharmacotherapy studies for the management of OA pain and cLBP, clinical recommendations from existing guidelines were adapted to fit into the Asian populations, by updating the recommendations with available evidence and incorporating the cultural, economic, and regulatory factors seen in the daily practice of physician/ pain specialists in Asian countries. The meeting was attended by eight experts from four Asian countries (Hong Kong, Japan, South Korea, and Taiwan) and one expert from Canada. During the meeting, we reviewed and discussed recent evidence and pharmacotherapy recommendations from existing guidelines, as well as current practice and local challenges in the pharmacological management of OA pain and cLBP.
A literature search was conducted via PubMed to identify recent systematic reviews, meta-analyses and available Asian randomized controlled trials (RCTs) on OA pain and cLBP published in English. The literature search period was based on the most recent available guidelines that are commonly referred to by the expert panel. For OA, the recent available guidelines are the 2014 National Institute for Health and Care Excellence (NICE) OA guidelines and thus, the search period used was January 2013 to April 2016. For LBP, the recent available guidelines are the 2011 Canadian guidelines and thus, the search period used was January 2010 to April 2016. From the search outcomes, relevant studies of chronic OA and LBP including treatment efficacy and safety, recent systematic reviews or meta-analyses, cost-effectiveness studies, and Asian RCTs were selected. Additional search for available Asian studies included keywords: Asian, Asia, China, Japan, Korea, or Taiwan. The exclusion criteria included non-clinical studies, specific LBP studies such as lumbar spinal stenosis, radiculopathy, and lumbar disc herniation, stem cells-related studies, epidemiology studies and studies of exploratory molecules. In the absence of or limited systematic reviews and meta-analyses, recent RCTs and comparative studies were included for review.
We reviewed relevant evidence and discussed our clinical experience and local factors that may affect the implementation of clinical recommendations in our daily practice. Following the development of the initial draft of the present manuscript, each of us reviewed the evidence for a pharmacological agent for the development of clinical recommendations. The subsequent draft incorporated all clinical recommendations, including the strength of recommendations based on the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system that classifies recommendations as either strong or weak.7 The draft was subjected to repeated detailed reviews to achieve complete consensus. Where evidence is conflicting or limited, we decided to make no recommendation until further evidence is available.
General Consideration
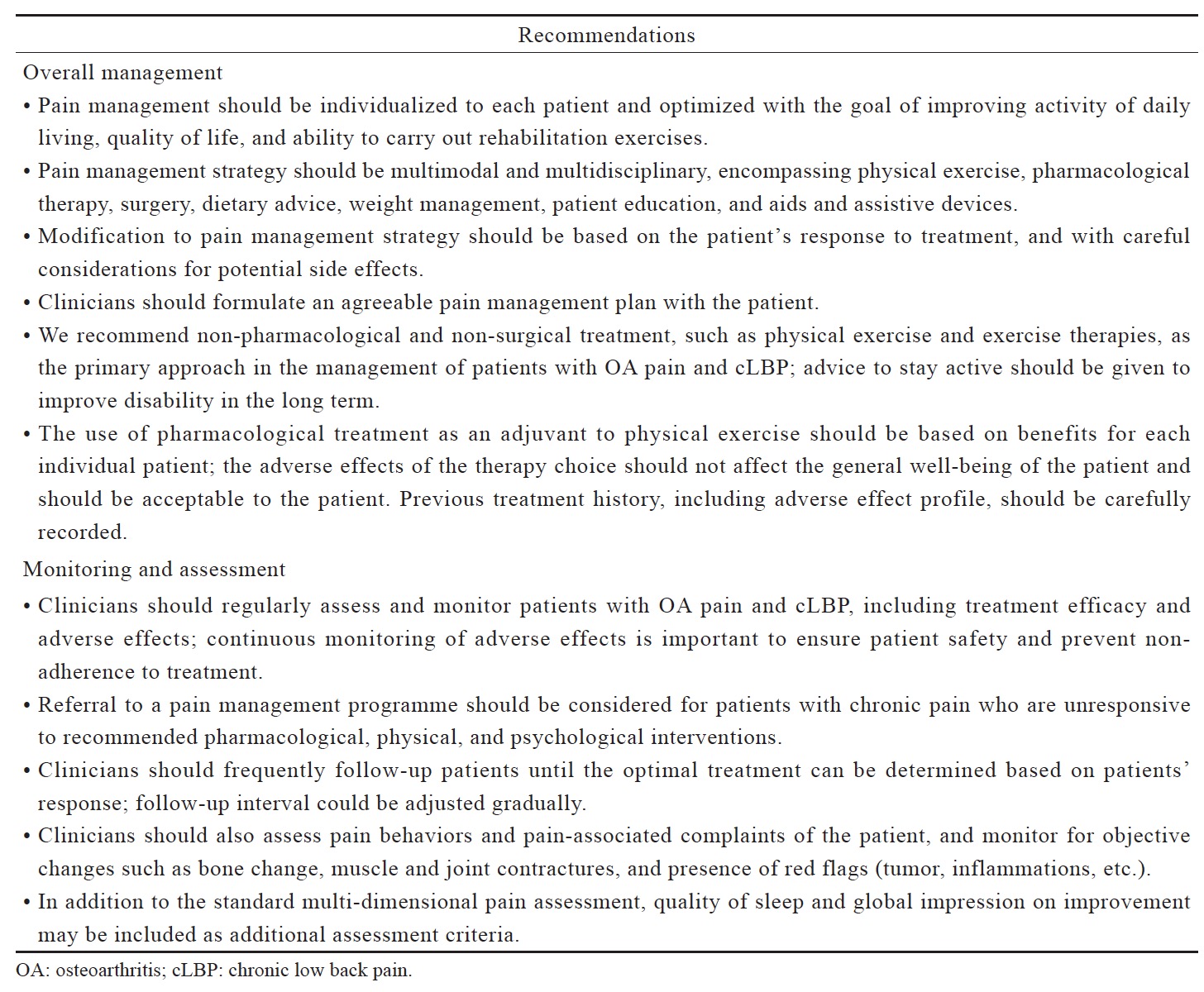
The primary goal of pain management is to control pain to a tolerable level that allows the person in pain to maximize activity and function. The optimal management of chronic musculoskeletal pain involves psychosocial factors, non-pharmacological (physical exercise, diet, education, managing mood and sleep disorders, etc.) and pharmacological treatments, given in consideration of the needs, risk factors and preferences of the individual. Based on our clinical experience, we provided general recommendations on the overall management, and the monitoring and assessment of OA pain and cLBP (Table 1).
Download full-size image
The complexity of pain necessitates a comprehensive biomedical, psychosocial and behavioral assessment for the proper management of patients with chronic musculoskeletal pain.8 Multi-dimensional pain assessment tools, such as the Brief Pain Inventory and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), are more useful for chronic pain patients as they capture the characteristics and quality of pain, satisfaction with pain control, and how pain affects mood and activities of daily living.9 Risks for cardiovascular disease, gastrointestinal (GI), renal and liver function need to be assessed when pharmacotherapies are considered. Drug selection and combination must consider the benefit against the potential risk for individual patient.
Follow-up assessment of patients with OA pain and cLBP is necessary but its frequency may be influenced by patient’s physical condition, patient’s transport/access to the clinics or hospitals, patient load in local clinics/hospitals in public or private sector, patient’s medical shopping, widespread use of alternative medicine, and financial and reimbursement issues. In Japan, the outpatient clinics provide more frequent follow-up than the hospitals; the typical follow-up frequency at the hospitals is once every 1–3 months. In Korea, smaller clinics provide more frequent follow-up compared with bigger hospitals. In Hong Kong, patient load determines the frequency of follow-up in the public sector whereas in the private sector, it is influenced by financial and reimbursement issues.
Pharmacological Agents for OA and cLBP
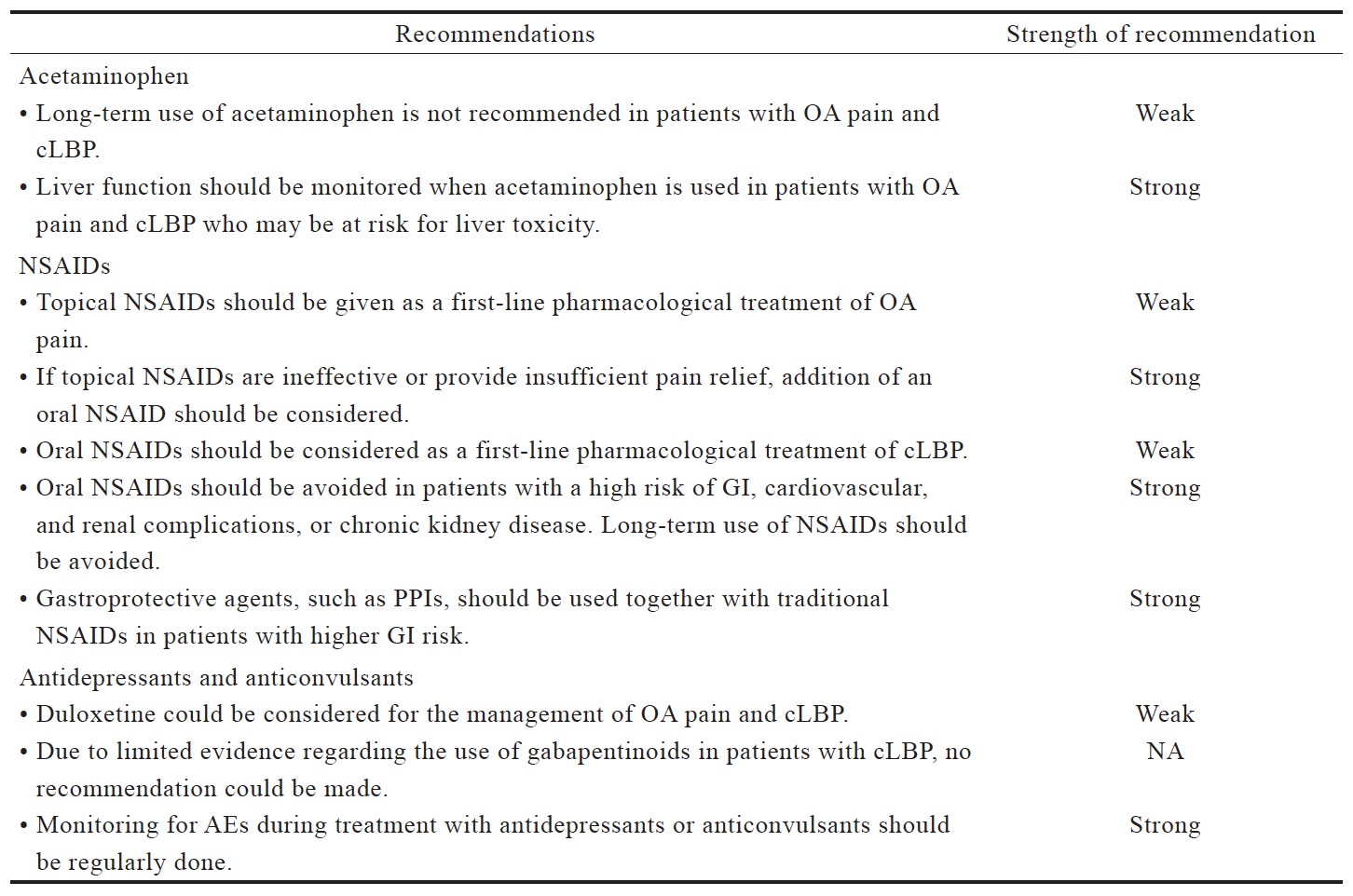
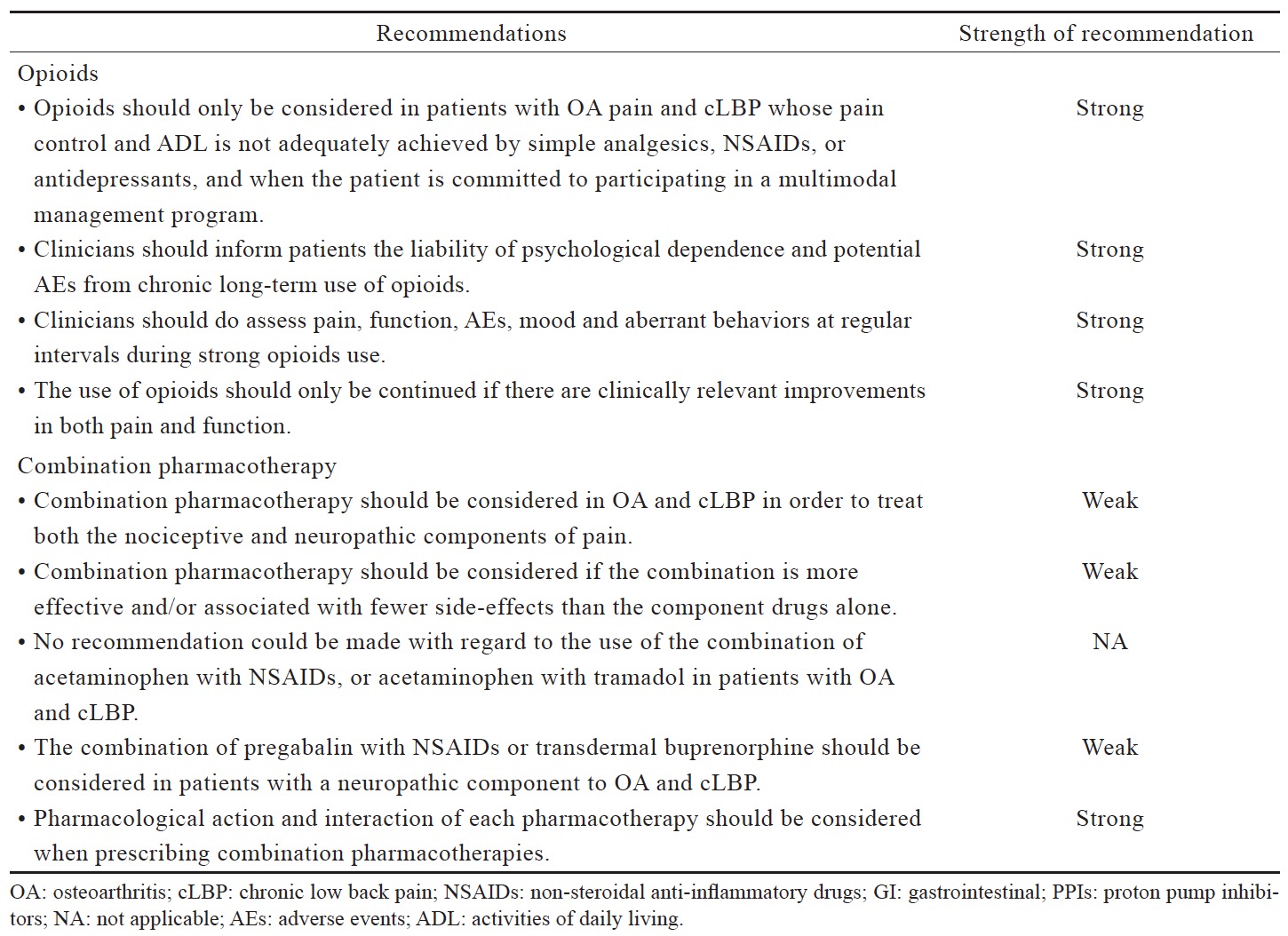
Table 2 lists the consensus recommendations for pharmacological management of OA pain and cLBP following our review of existing guidelines (Table 3) and available evidence (Suppl. Tables 1–5), which were summarized below. These recommendations serve only as a guide for decision-making in clinical practice and are not prescriptive. As such, these recommendations would not necessarily apply to all patients with OA pain and cLBP.
Download full-size image
Download full-size image
There are several unanswered issues pertaining to chronic pain management, including the lack of long-term studies of pharmacotherapies, when to change pain medication strategy, and availability of other treatment options for patients who do not respond to any of the recommended treatments.
Acetaminophen
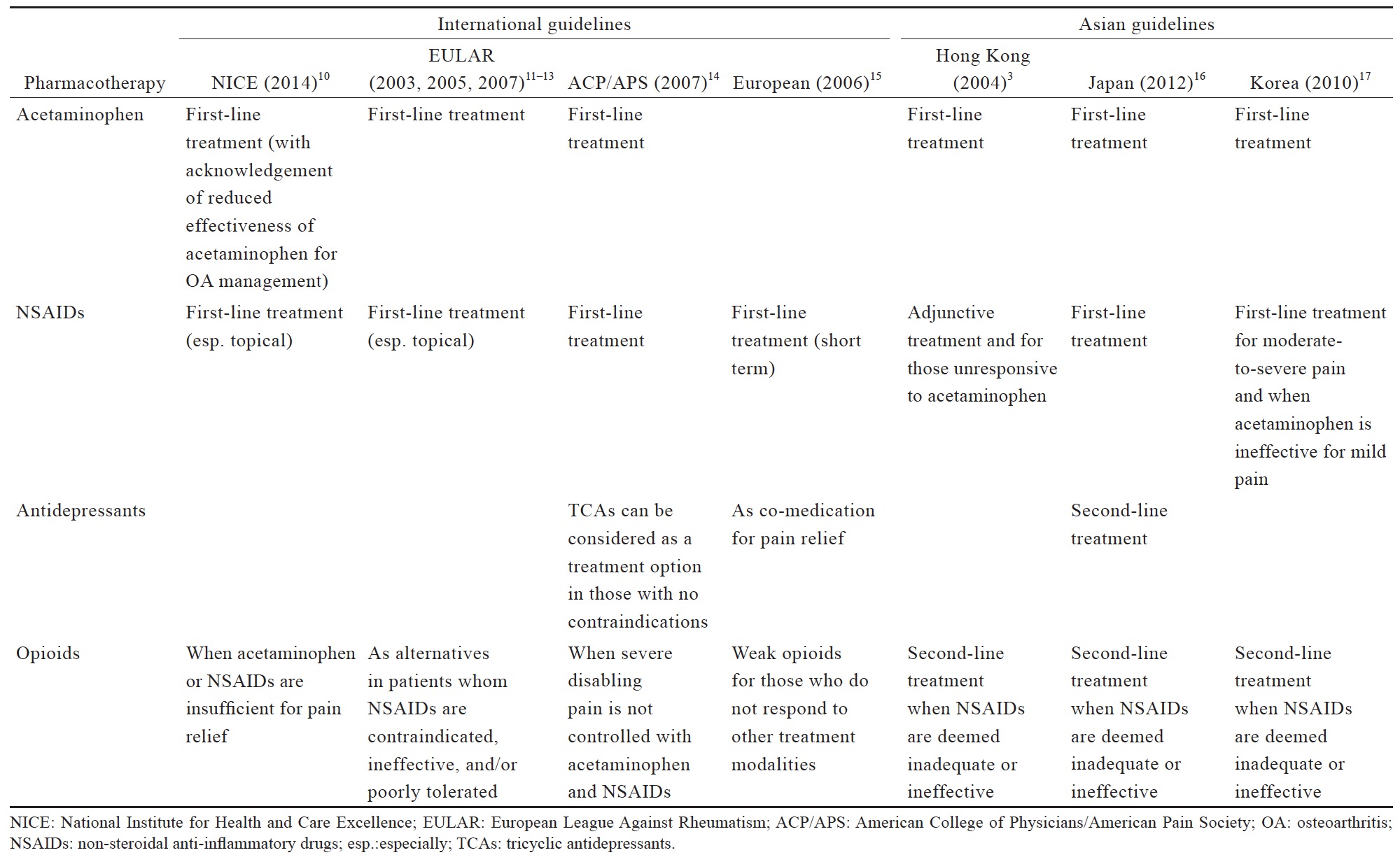
The existing international and Asian guidelines for chronic pain management have consistently recommended acetaminophen as a first-line analgesic (Table 3). The 2014 NICE guidelines for OA management maintain the recommended use of acetaminophen ahead of other oral pharmacotherapies, but also note that there is evidence for a reduced effectiveness of acetaminophen in the management of OA, which should be taken into account in routine prescribing practice.10
Download full-size image
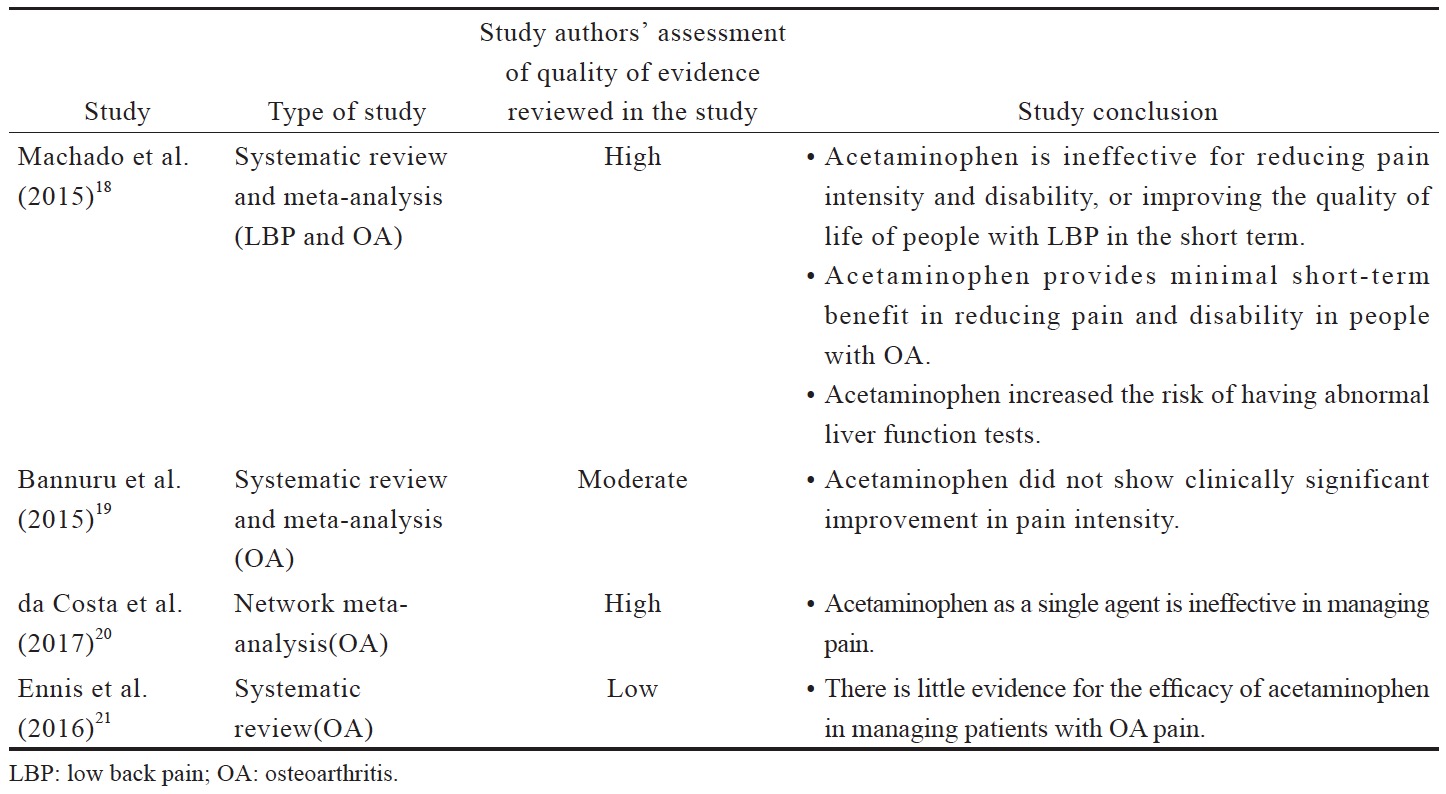
Despite being recommended for first-line use, recent data suggests that there is high quality evidence that acetaminophen is ineffective for managing pain and improving the quality of life of people with LBP and OA (Suppl. Table 1).18-21 The short treatment duration in these evaluated trials (often up to 6 weeks, with up to 12 weeks of follow-ups) does not allow evaluation of the benefit versus safety of long-term use of acetaminophen.
Although the safety and efficacy of acetaminophen has not been evaluated in Asian populations, the findings of available studies clearly contradict the long-standing recommendation for acetaminophen use as a first-line analgesic in managing chronic pain, as well as the current clinical practice of Asian doctors. Acetaminophen should no longer be recommended as a first-line analgesic based on the lack of efficacy; however, it can be used in specific patient populations, in whom other analgesics may not be suitable.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are often recommended as a first-line pharmacological agent for OA and LBP management in international and Asian guidelines, with the exception of the Hong Kong clinical guidelines (Table 3). These guidelines generally recommend assessment of patient’s cardiovascular and GI risk prior to prescribing NSAIDs, and short duration use of NSAIDs. Co-prescription of gastro-protective agents are also recommended (Table 3).
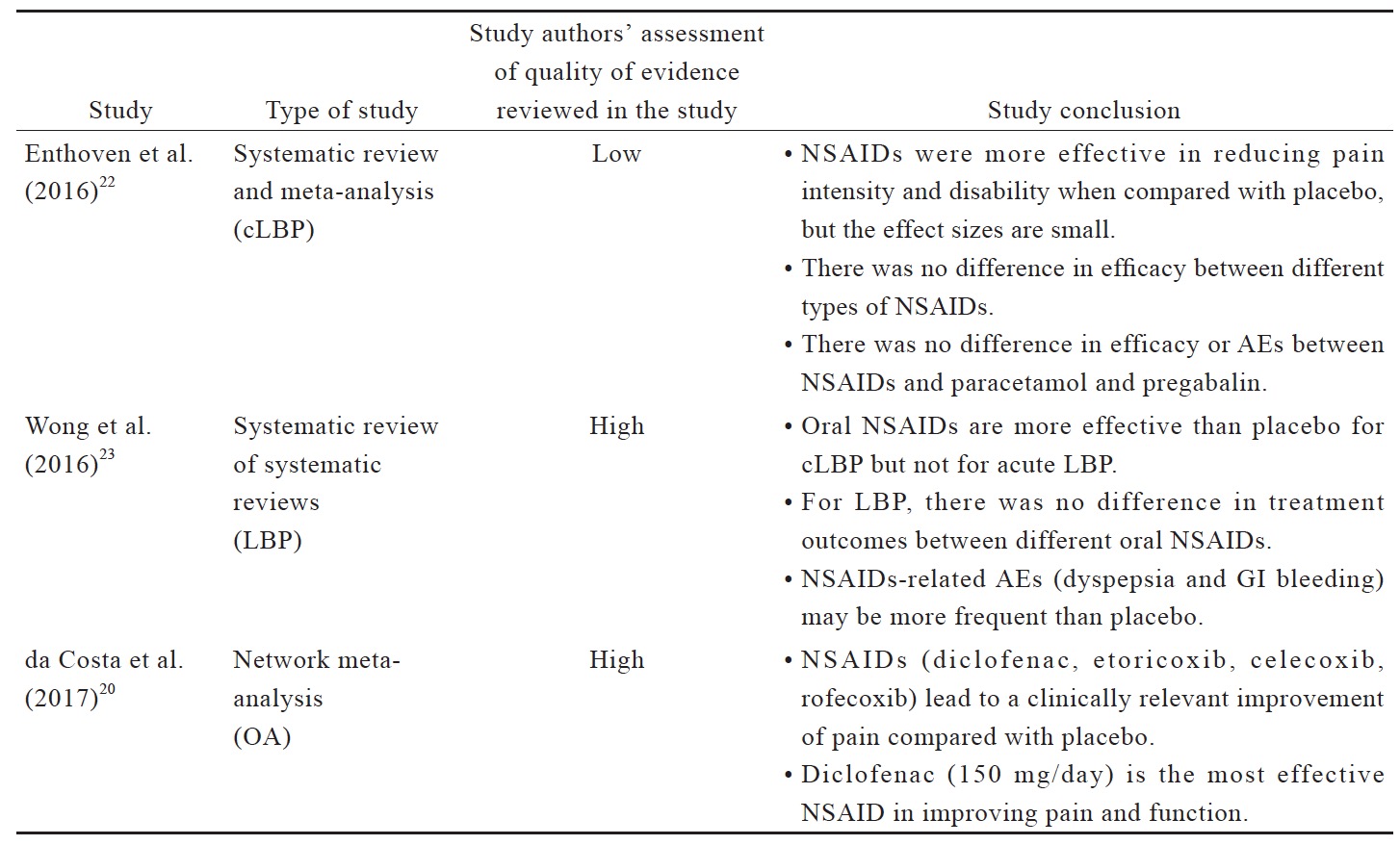
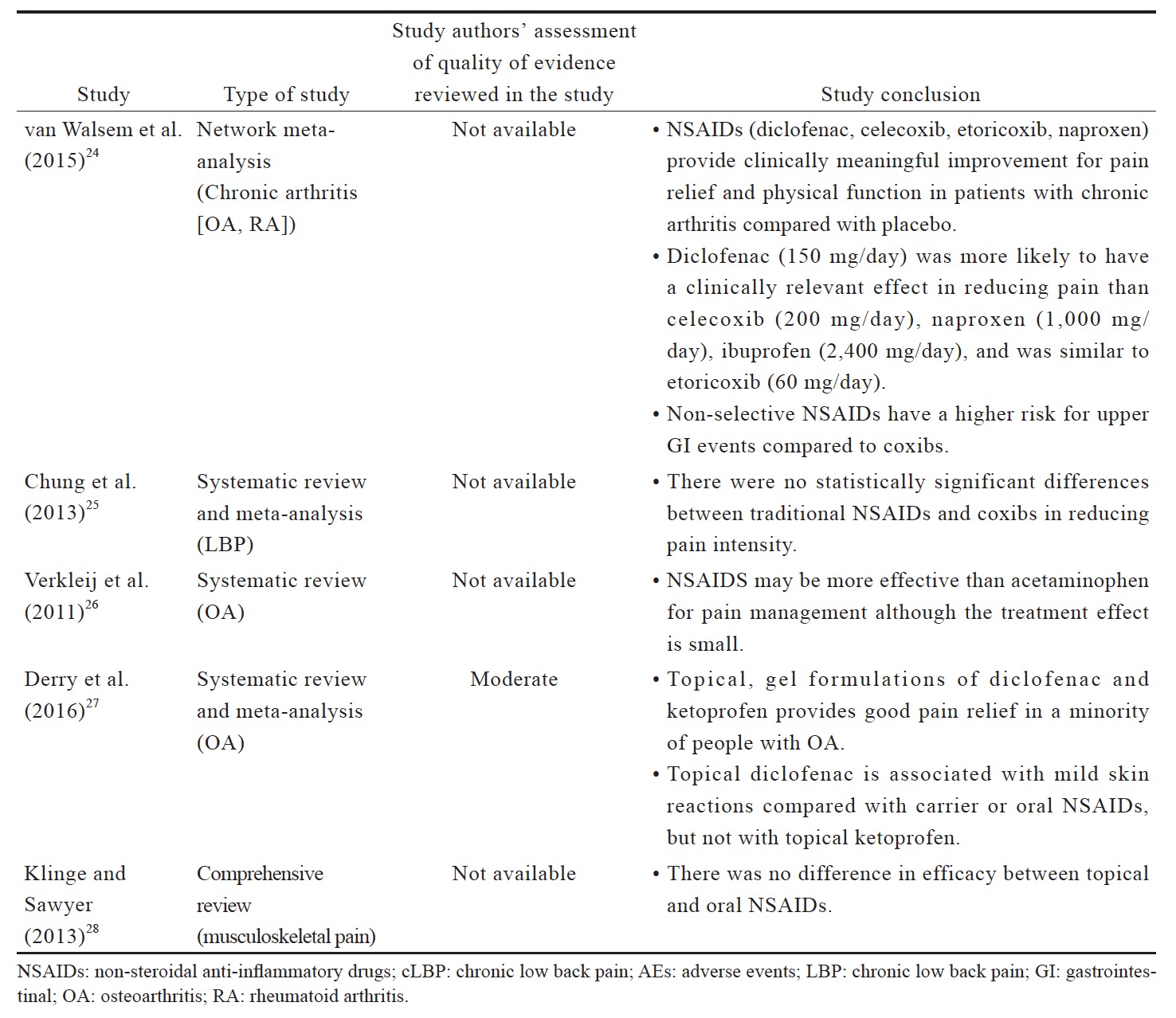
Based on the evidence reviewed (Suppl. Table 2),20,22-28 NSAIDs are more effective than placebo in OA and LBP management. Although there is no evidence for superiority of a particular type of NSAID over the others for LBP, diclofenac and etoricoxib are more likely to be more effective for OA. There is no evidence that NSAIDs are more effective than acetaminophen for LBP but there is a small effect favoring NSAIDs for OA. Topical NSAIDs provide good pain relief in OA, and their efficacies are comparable with oral NSAIDs for musculoskeletal pain. In terms of safety, non-selective NSAIDs have a higher risk for upper GI events compared with coxibs; there is also evidence for an increase in local adverse events (AEs) with the use of topical NSAIDs. However, these systematic reviews and meta-analyses are not able to address the issues pertaining to long-term use of NSAIDs.20,22 Head-to-head long-term trials are required for a definite conclusion on the long-term safety of NSAIDs. Intermittent short-term use of NSAIDs in moderate to maximum doses can be considered as required to balance the benefit and potential risk.20
Antidepressants and Anticonvulsants
Antidepressants are typically recommended as an alternative or adjuvant option in the guidelines for LBP management (Table 3),14,15 with no recommendations for anticonvulsant use. In the Japanese LBP guidelines, antidepressants could be considered as second-line medication.16 Both antidepressants and anticonvulsants are not recommended in OA guidelines.
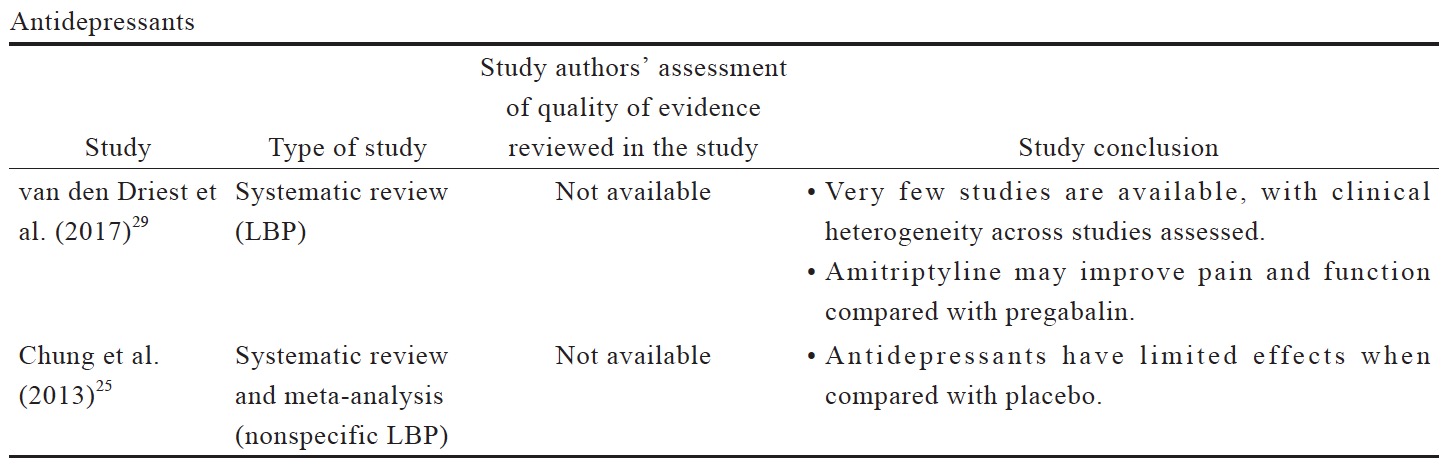
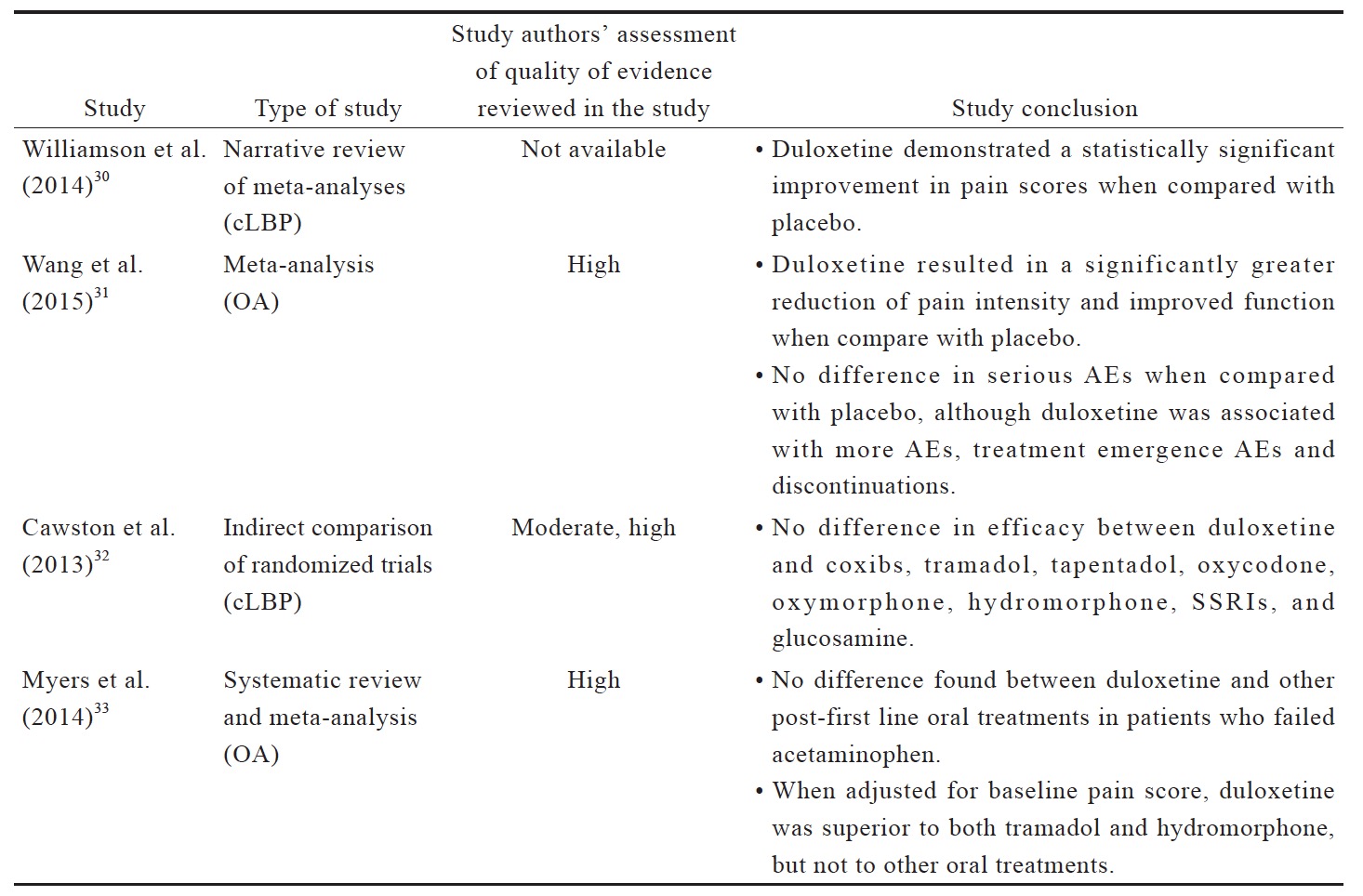
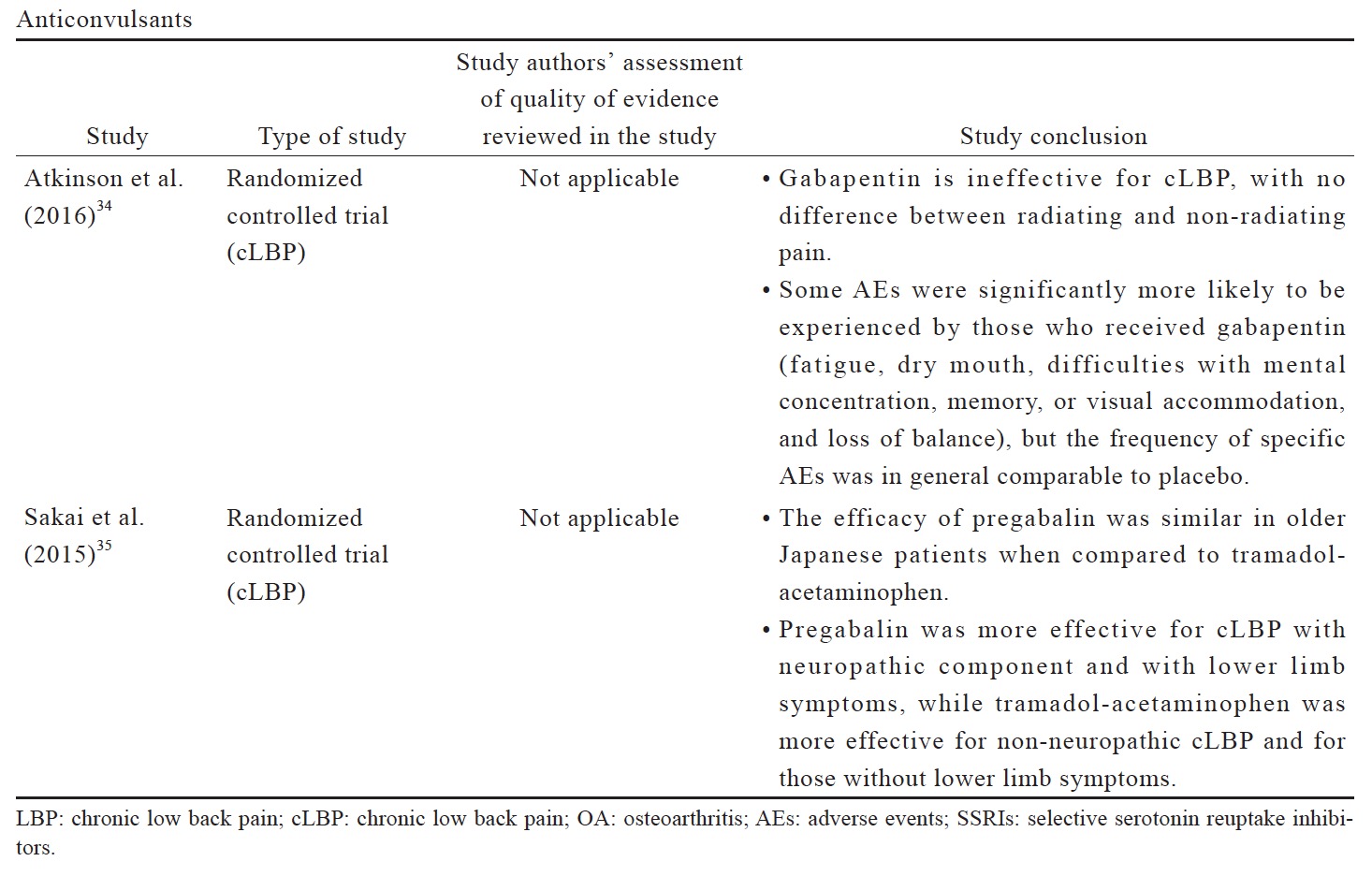
The data reviewed (Suppl. Table 3)25,29-35 indicates that there is limited evidence for duloxetine in reducing pain intensity for OA pain and LBP when compared to placebo or other oral pharmacotherapies. Compared to placebo, duloxetine was associated with more AEs, including nausea, constipation, dry mouth, diarrhea, fatigues, dizziness, somnolence, and insomnia.31 Although anticonvulsant gabapentin is prescribed as an analgesic in cLBP, trials supporting this practice are limited. There is conflicting evidence for the efficacy of gabapentinoids for cLBP with associated radicular symptoms. While there are a number of studies of anticonvulsants in cLBP management, their use in OA pain management are not well studied.
Opioids
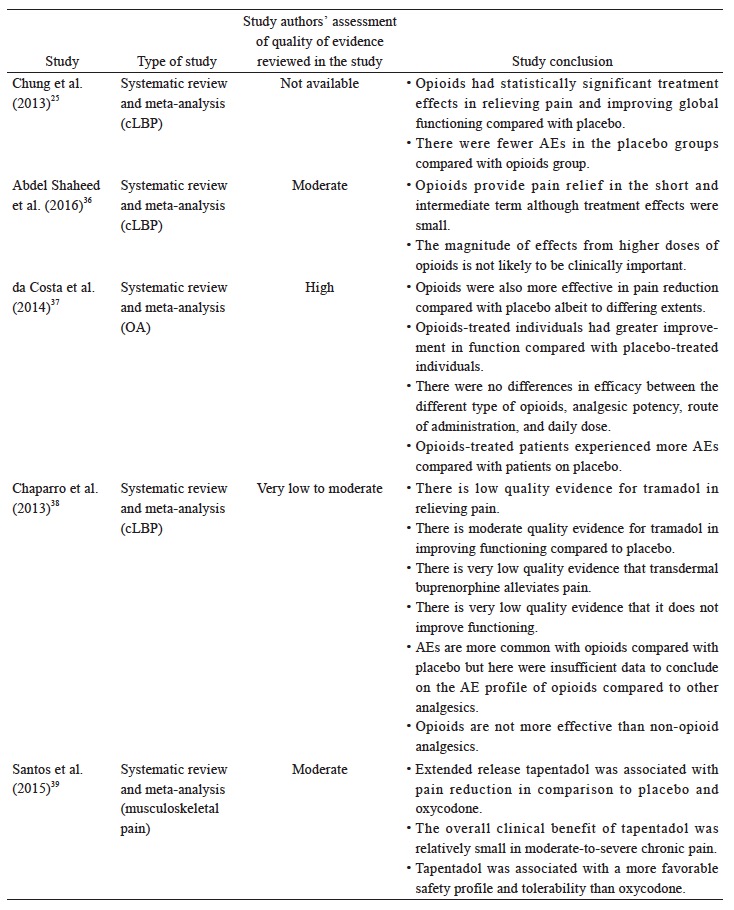
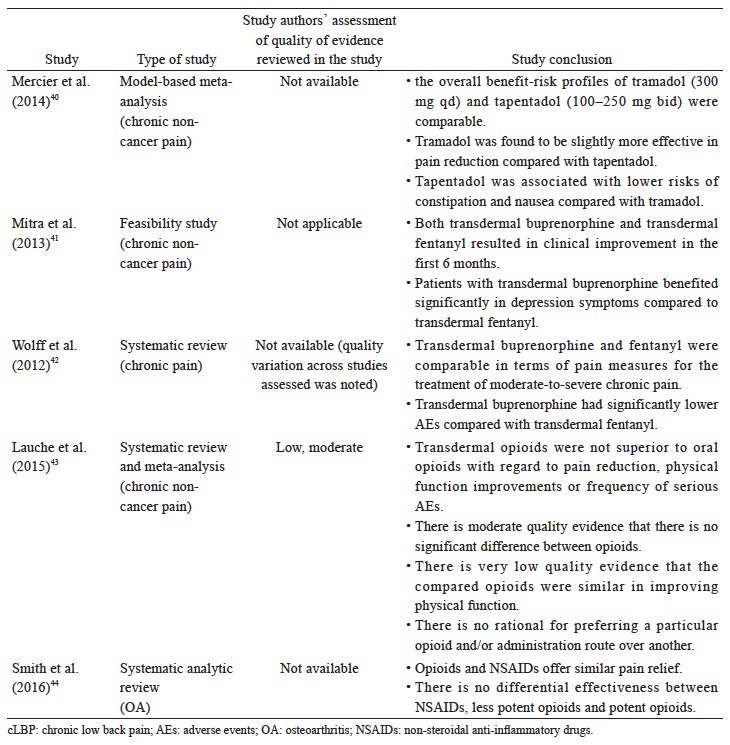
The recommended use of opioids in the currently available guidelines is generally reserved for when severe disabling pain is not controlled with acetaminophen, NSAIDs, or other treatment modalities, or when NSAIDs are contraindicated (Table 3). The Asian guidelines recommend opioids as second-line therapy for cLBP and OA when NSAIDs are deemed inadequate or ineffective (Table 3). Based on the available data (Suppl. Table 4),25,36-44 there is strong evidence that opioids are effective in alleviating pain and improving function in OA and LBP patients; however, with higher incidence of AEs than placebo. Opioids may not be more effective compared with other analgesics. While tramadol and tapentadol are similar in terms of efficacy, their clinical benefit is relatively small. There is limited evidence that the transdermal formulation of opioids offers several advantages compared to the oral formulation. There is no evidence to support the use of one opioid over another with regard to efficacy, route of administration and doses. Opioids-treated patients had significantly higher incidence of AEs compared with patients on placebo. Up to date, evidence for longterm use of opioids at any dose in chronic non-malignant pain remains insufficient to determine long-term benefits, highlighting the need for such trials.
Combination Pharmacotherapy
Available guidelines generally recommend the use of pharmacotherapy combinations when prior treatment is inadequate or ineffective but do not recommend specific combinations. The 2014 NICE guidelines for OA management recommend the addition of stronger analgesics when prior treatments are insufficient based on risks and benefits consideration, but also acknowledges that there is limited evidence on the effects of combination therapies.10 Likewise, the Hong Kong OA guidelines consider the combination of opioid analgesics and acetaminophen as an option for the treatment of moderate-to-severe pain in patients who are tolerant or unresponsive to NSAIDs.3 By contrast, the use of combination pharmacotherapy is not included in LBP guidelines.14,15
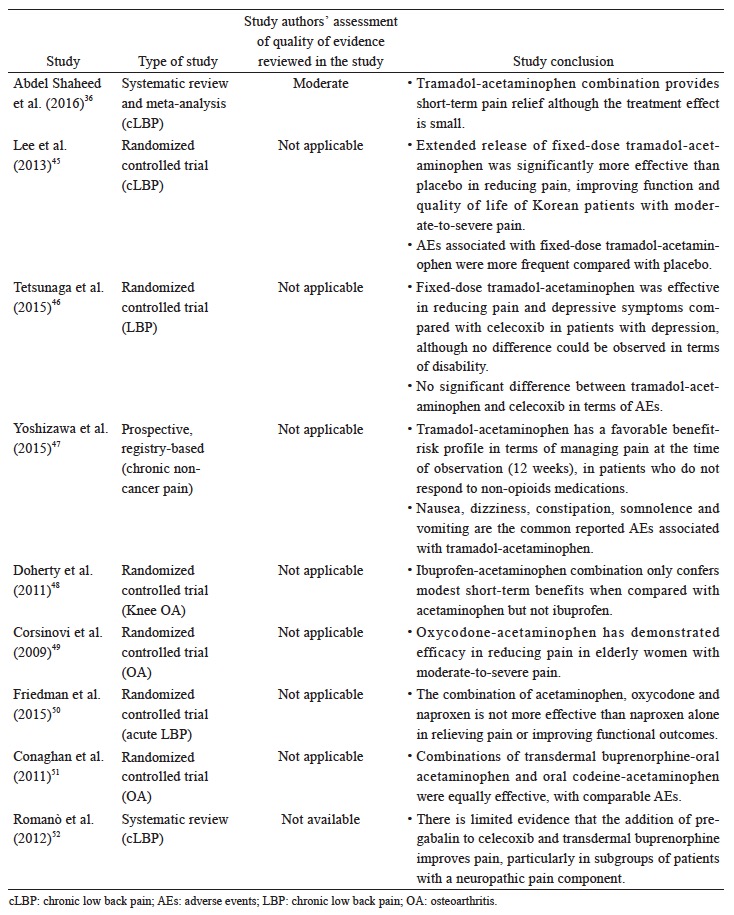
The data reviewed (Suppl. Table 5)36,45-52 suggest that there is limited evidence to support the use of a specific combination pharmacotherapy in OA and cLBP. The recent systematic review and meta-analysis by Abdel Shaheed et al.,36 which included primarily western studies, indicates weak evidence that the combination of acetaminophen and tramadol is not more effective than tramadol alone in improving pain and function, but recent studies in Asian populations seem to demonstrate efficacy although the clinical magnitude of the effect remains to be determined.
Implementation in Asia
The implementation of these recommendations (Table 2) in each country may be influenced by culture, local regulations, reimbursement/cost issues, and patient’s preference.
Acetaminophen Use
Hepatotoxicity is a key concern in the region due to the prevalence of chronic hepatitis B infection which is estimated to range between 1.02% in Japan to 5.49% in China.53 Despite so, the overall prevalence of actual liver toxicity due to acetaminophen is reported to be generally low (7.3%), thus acetaminophen is still used as first-line treatment in the region.54
While the incidence of acetaminophen-associated hepatotoxicity is low, we noted that there is a lack of patient awareness of the potential risks of high dose or long-term acetaminophen use in the region. Asians tend to underestimate their pain experience, are reluctant to report their complaints, and do not want to spend too much money on treatment. These may prevent them from seeking appropriate treatment and lead to self-medication. Patients may be taking medications at inappropriate dose or in combination with other medications or herbal remedies that may increase the risk of hepatotoxicity.
Acetaminophen use in the region is also influenced by local regulations. For example, the new chronic kidney disease (CKD) guidelines in Japan prohibit the use of NSAIDs in patients at risk for CKD, which leads to the use of acetaminophen in this subgroup of patients.
NSAIDs Use
We noted that there is a preference for the use of NSAID patches in the region. The simpler and non-invasive application, along with the advantage of reduction in systemic AEs, renders NSAID patches a favorable treatment option for patients.
Local guidelines also influence the use of NSAIDs in the region—hospital guidelines in Hong Kong recommend oral over topical NSAIDs, and patients with higher GI risk tend to be prescribed with non-specific NSAIDs in combination with a proton pump inhibitor. In Korea, coxibs are prescribed for higher-risk patients.
Opioids Use
We agreed that there is a need for more high quality trials on opioids use in cLBP and OA patients, with clear inclusion and exclusion criteria and objectives, such as improvement in pain and activities of daily living as well as long-term safety. These will help to provide justification for opioids use in chronic musculoskeletal pain management, and allow eligible patients to benefit from this treatment option.
Local and international guidelines for the use of opioids in chronic non-cancer pain are available, including the 2012 Guidelines for Prescribing Opioid Analgesics for Chronic Non-Cancer Pain published by the Japanese Society of Pain Clinicians55; the 2016 Opioid Therapy for Chronic Non-Cancer Pain: Guidelines for Hong Kong published by the Hong Kong Hospital Authority Multidisciplinary Committee on Pain Medicine56; the 2017 Guidelines for Prescribing Opioids for Chronic Non-Cancer Pain in Korea published by the Opioid Research Group in the Korean Pain Society57 58 ; and the 2017 Canadian guideline for Opioids for Chronic Non-Cancer Pain by the National Pain Centre of McMaster University, Canada.57 58 Our recommendations on opioid use, while not specific, are consistent with these guidelines.
There is a general concern and fear of addiction in the region that lead to very restrictive policies limiting opioid use for chronic non-cancer pain. In Taiwan, prescription for more than 2 weeks must follow the national guidelines—the process involves three independent interdisciplinary assessments, evaluation of the three assessments by the independent members of a hospital committee, and final approval by the controlled drug committee who conducts review every 4 months. All patients have to be re-evaluated every 6 months. In Japan, patients are reluctant to take opioids due to negative perceptions associated with its use after World War II. There is also no proper system to control or limit the prescription of strong opioids, although they are prescribed as a second-line therapy for cLBP in Japan. Hence, a balanced system that allows for safe prescribing of opioids for chronic musculoskeletal pain management is needed. Patients in Korea are also concerned over the risk of addiction and AEs of opioid, and clinicians have insufficient experience in prescribing opioids. In Hong Kong, opioids are not widely used for chronic musculoskeletal pain treatment due to inadequate knowledge of the pharmacological properties and indications amongst clinicians, and a lack of local guidelines on early use of opioids. Resources to support the continuous monitoring of opioids use are limited in the public sector and thus, opioids are generally avoided due to AEs and concerns over risk of abuse. Additionally, opioids are relatively more expensive in comparison to simple analgesics or NSAIDs, thus clinicians would defer early use of the more expensive opioids.
Conclusion
We developed a set of consensus recommendations for the pharmacological management of OA pain and cLBP (Tables 1 and 2) based on the review of existing guidelines (Table 3), newer evidence (Suppl. Tables 1–5) and expert discussion on current practice and challenges in the Asian region. As part of overall pain management, we agreed that pain management strategy should be tailored to each individual patient, and non-pharmacological treatment, such as exercise, should be the primary approach before considering pharmacotherapy. Regular pain assessment and monitoring during any treatment approach is essential. Where evidence is conflicting or limited, we decided to make no recommendation until further evidence is available.
The implementation of these consensus recommendations in each country will vary depending on cultural and regulatory factors. There are various challenges in different countries in the Asian region pertaining to the use of pharmacological agents in the management of OA pain and cLBP, particularly opioids. A better understanding and knowledge of opioids is thus necessary to improve the pain control of patients with OA and cLBP.
Ultimately, clinicians should take into account the nature and severity of pain, benefit versus risk profile of pharmacotherapy and combination pharmacotherapy, as well as patient preference, when determining the optimal pharmacotherapy in the management of OA pain and cLBP.
Acknowledgments
The authors thank Yulyana of In Vivo Communications (Asia) Pte Ltd. for the provision of medical writing assistance in the preparation of this manuscript.
Author Contributions
All authors participated in the consensus meeting, made substantial contributions to the review of evidence and formulation of the consensus statements, preparation of the manuscript, provided critical revision for intellectual content and final approval for submission.
Funding
This work was supported by an independent grant from Mundipharma Pte Ltd. The funder had no role in determining the content of the consensus or in manuscript preparation.
Declaration
All of the authors received honoraria and travel funding from Mundipharma Pte Ltd. to attend the consensus meeting. Dr. Owen D Williamson has received honoraria from Purdue Canada. Dr. Takahiro Ushida has received consulting fee from Daiichi Sankyo, Pfizer, Taisho Toyama, Mundipharma, Eli-Lilly Japan, Showa Yakuhin, Sumitomo Dainippon Pharma, and Janssen Japan; and research grants from Nihon Zoki, Seikagaku Kogyo, Pfizer, Daiichi Sankyo, Tsumura, Shionogi, Eli-Lilly Japan, Astellas, Eisai, and Chugai.
References
| 1 |
Treede RD, Rief W, Barke A, et al.
A classification ofchronic pain for ICD-11.
Pain 2015;156:1003–1007.
|
| 2 |
Clauw DJ.
Diagnosing and treating chronic musculoskeletalpain based on the underlying mechanism(s).
BestPract Res Clin Rheumatol 2015;29:6–19.
|
| 3 |
Chinese University of Hong Kong.
Clinical guidelines formanaging lower-limb osteoarthritis in Hong Kong primarycare setting.
Hong Kong: Department of Communityand Family Medicine, Chinese University of Hong Kong; 2004.
|
| 4 |
Cheng L, Lau KKS, Lam WK, et al.
Evidence-basedguideline on prevention and management of low backpain in working population in primary care.
HK Pract2012;34:106–115.
|
| 5 |
Akaza H.
What is the Asian Consensus Statement onNCCN clinical practice guidelines in oncology (NCCNACS)?
Jpn J Clin Oncol 2016;46:299–302.
|
| 6 |
Ho PC, Johnson MH.
Behaviours and beliefs about painand treatment among Chinese immigrants and New ZealandEuropeans.
N Z Med J 2013;126:10–22.
|
| 7 |
Andrews J, Guyatt G, Oxman AD, et al.
GRADE guidelines: 14. Going from evidence to recommendations:the significance and presentation of recommendations.
J Clin Epidemiol 2013;66:719–725.
|
| 8 |
Turk DC, Fillingim RB, Ohrbach R, Patel KV.
Assessmentof psychosocial and functional impact of chronic pain.
JPain 2016;17:T21–T49.
|
| 9 |
Wellington B, Flynn S, Duperouzel W, Treloar S.
Assessmentof chronic pain: a practice update.
Int JOrthop Trauma Nurs 2015;19:155–161.
|
| 10 |
National Clinical Guideline Centre.
Osteoarthritis:care and management in adults.
National Institute forHealth and Care Excellence. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0068962/pdf/PubMedHealth_PMH0068962.pdf.. Accessed June 16, 2016.
|
| 11 |
Jordan KM, Arden NK, Doherty M, et al.
EULAR recommendations2003: an evidence based approach tothe management of knee osteoarthritis: report of atask force of the Standing Committee for InternationalClinical Studies Including Therapeutic Trails (ESCISIT).
Ann Rheum Dis 2003;62:1145–1155.
|
| 12 |
Zhang W, Doherty M, Arden N, et al.
EULAR evidencebased recommendations for the management of hip osteoarthritis:report of a task force of the EULAR StandingCommittee for International Clinical Studies IncludingTherapeutics (ESCISIT).
Ann Rheum Dis 2005;64:669–681.
|
| 13 |
Zhang W, Doherty M, Leeb BF, et al.
EULAR evidencebased recommendations for the management of handosteoarthritis: report of a Task Force of the EULARStanding Committee for International Clinical StudiesIncluding Therapeutics (ESCISIT).
Ann Rheum Dis2007;66:377–388.
|
| 14 |
Chou R, Qaseem A, Snow V, et al.
Diagnosis and treatmentof low back pain: a joint clinical practice guidelinefrom the American College of Physicians and the AmericanPain Society.
Ann Intern Med 2007;147:478–491.
|
| 15 |
Airaksinen O, Brox JI, Cedraschi C, et al.
Chapter 4 Europeanguidelines for the management of chronic nonspecificlow back pain.
Eur Spine J 2006;15(Suppl 2):S192–S300.
|
| 16 |
Japanese Orthopaedic Association [Nihon seikeigekagakkai].
Low back pain clinical practice guidelines 2012[Yotsu shinryo gaidorain 2012].
Tokyo: Nankodo Co.,Ltd.; 2012.
|
| 17 |
Korean Knee Society Subcommittee on OsteoarthritisGuidelines.
Guidelines for the treatment of osteoarthritisof the knee.
J Korean Knee Soc 2010;22:69–74.
|
| 18 |
Machado GC, Maher CG, Ferreira PH, et al.
Efficacy andsafety of paracetamol for spinal pain and osteoarthritis:systematic review and meta-analysis of randomized placebocontrolled trials.
BMJ 2015;350:h1225.
|
| 19 |
Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, WongJB, McAlindon TE.
Comparative effectiveness of pharmacologicinterventions for knee osteoarthritis: a systematicreview and network meta-analysis.
Ann Intern Med2015;162:46–54.
|
| 20 |
da Costa BR, Reichenbach S, Keller N, et al.
Effectivenessof non-steroidal anti-inflammatory drugs for the treatmentof pain in knee and hip osteoarthritis: a networkmeta-analysis.
Lancet 2017;390:e21–e33.
|
| 21 |
Ennis ZN, Dideriksen D, Vaegter HB, Handberg G,Pottegård A.
Acetaminophen for chronic pain: a systematicreview on efficacy.
Basic Clin Pharmacol Toxicol2016;118:184–189.
|
| 22 |
Enthoven WT, Roelofs PD, Deyo RA, van Tulder MW,Koes BW.
Non-steroidal anti-inflammatory drugs forchronic low back pain.
Cochrane Database Syst Rev2016;2:CD012087.
|
| 23 |
Wong JJ, Côté P, Ameis A, et al.
Are non-steroidal anti-inflammatorydrugs effective for the management of neckpain and associated disorders, whiplash-associated disorders,or non-specific low back pain? A systematic reviewof systematic reviews by the Ontario Protocol for TrafficInjury Management (OPTIMa) Collaboration.
Eur SpineJ 2016;25:34–61.
|
| 24 |
van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A,Moore RA.
Relative benefit-risk comparing diclofenac toother traditional non-steroidal anti-inflammatory drugsand cyclooxygenase-2 inhibitors in patients with osteoarthritisor rheumatoid arthritis: a network meta-analysis.
Arthritis Res Ther 2015;17:66.
|
| 25 |
Chung JW, Zeng Y, Wong TK.
Drug therapy for the treatmentof chronic nonspecific low back pain: systematicreview and meta-analysis.
Pain Physician 2013;16:E685–E704.
|
| 26 |
Verkleij SP, Luijsterburg PA, Bohnen AM, Koes BW, Bierma-Zeinstra SM.
NSAIDs vs acetaminophen in knee andhip osteoarthritis: a systematic review regarding heterogeneityinfluencing the outcomes.
Osteoarthritis Cartilage2011;19:921–929.
|
| 27 |
Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA.
Topical NSAIDs for chronic musculoskeletal pain inadults.
Cochrane Database Syst Rev 2016;4:CD007400.
|
| 28 |
Klinge SA, Sawyer GA.
Effectiveness and safety of topicalversus oral nonsteroidal anti-inflammatory drugs: acomprehensive review.
Phys Sportsmed 2013;41:64–74.
|
| 29 |
van den Driest JJ, Bierma-Zeinstra SMA, Bindels PJE,Schiphof D.
Amitriptyline for musculoskeletal complaints:a systematic review.
Fam Pract 2017;34:138–146.
|
| 30 |
Williamson OD, Sagman D, Bruins RH, Boulay LJ,Schacht A.
Antidepressants in the treatment for chroniclow back pain: questioning the validity of meta-analyses.
Pain Pract 2014;14:E33–E41.
|
| 31 |
Wang ZY, Shi SY, Li SJ, et al.
Efficacy and safety of duloxetineon osteoarthritis knee pain: a meta-analysis of randomizedcontrolled trials.
Pain Med 2015;16:1373–1385.
|
| 32 |
Cawston H, Davie A, Paget MA, Skljarevski V, HappichM.
Efficacy of duloxetine versus alternative oral therapies:an indirect comparison of randomised clinical trials inchronic low back pain.
Eur Spine J 2013;22:1996–2009.
|
| 33 |
Myers J, Wielage RC, Han B, et al.
The efficacy of duloxetine,non-steroidal anti-inflammatory drugs, and opioidsin osteoarthritis: a systematic literature review andmeta-analysis.
BMC Musculoskelet Disord 2014;15:76.
|
| 34 |
Atkinson JH, Slater MA, Capparelli EV, et al.
A randomizedcontrolled trial of gabapentin for chronic low back pain withand without a radiating component.
Pain 2016;157:1499–1507.
|
| 35 |
Sakai Y, Ito K, Hida T, Ito S, Harada A.
Pharmacologicalmanagement of chronic low back pain in older patients: arandomized controlled trial of the effect of pregabalin andopioid administration.
Eur Spine J 2015;24:1309–1317.
|
| 36 |
Abdel Shaheed C, Maher CG, Williams KA, Day R,McLachlan AJ.
Efficacy, tolerability, and dose-dependenteffects of opioid analgesics for low back pain: asystematic review and meta-analysis.
JAMA Intern Med2016;176:958–968.
|
| 37 |
da Costa BR, Nüesch E, Kasteler R, et al.
Oral or transdermalopioids for osteoarthritis of the knee or hip.
CochraneDatabase Syst Rev 2014;9:CD003115.
|
| 38 |
Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A,Atlas S, Turk DC.
Opioids compared to placebo or othertreatments for chronic low-back pain.
Cochrane DatabaseSyst Rev 2013;8:CD004959.
|
| 39 |
Santos J, Alarcão J, Fareleira F, Vaz-Carneiro A, CostaJ.
Tapentadol for chronic musculoskeletal pain inadults.
Cochrane Database Syst Rev 2015;5:CD009923.
|
| 40 |
Mercier F, Claret L, Prins K, Bruno R.
A model-based meta-analysis to compare efficacy and tolerability of tramadoland tapentadol for the treatment of chronic non-malignantpain.
Pain Ther 2014;3:31–44.
|
| 41 |
Mitra F, Chowdhury S, Shelley M, Williams G.
A feasibilitystudy of transdermal buprenorphine versus transdermalfentanyl in the long-term management of persistentnon-cancer pain.
Pain Med 2013;14:75–83.
|
| 42 |
Wolff RF, Aune D, Truyers C, et al.
Systematic review ofefficacy and safety of buprenorphine versus fentanyl ormorphine in patients with chronic moderate to severepain.
Curr Med Res Opin 2012;28:833–845.
|
| 43 |
Lauche R, Klose P, Radbruch L, Welsch P, Häuser W.
Opioids in chronic noncancer pain—are opioids different?A systematic review and meta-analysis of efficacy,tolerability and safety in randomized head-to-headcomparisons of opioids of at least four week’s duration.
Schmerz 2015;29:73–84. [In German, English abstract]
|
| 44 |
Smith SR, Deshpande BR, Collins JE, Katz JN, LosinaE.
Comparative pain reduction of oral non-steroidalanti-inflammatory drugs and opioids for knee osteoarthritis:systematic analytic review.
Osteoarthritis Cartilage2016;24:962–972.
|
| 45 |
Lee JH, Lee CS, Ultracet ER Study Group.
A randomized,double-blind, placebo-controlled, parallel-group studyto evaluate the efficacy and safety of the extended-releasetramadol hydrochloride/acetaminophen fixed-dosecombination tablet for the treatment of chronic low backpain.
Clin Ther 2013;35:1830–1840.
|
| 46 |
Tetsunaga T, Tetsunaga T, Tanaka M, Ozaki T.
Efficacyof tramadol-acetaminophen tablets in low back painpatients with depression.
J Orthop Sci 2015;20:281–286.
|
| 47 |
Yoshizawa K, Kawai K, Fujie M, et al.
Overall safetyprofile and effectiveness of tramadol hydrochloride/acetaminophen in patients with chronic noncancer painin Japanese real-world practice.
Curr Med Res Opin2015;31:2119–2129.
|
| 48 |
Doherty M, Hawkey C, Goulder M, et al.
A randomizedcontrolled trial of ibuprofen, paracetamol or a combinationtablet of ibuprofen/paracetamol in community-derived people with knee pain.
Ann Rheum Dis2011;70:1534–1541.
|
| 49 |
Corsinovi L, Martinelli E, Fonte G, et al.
Efficacy of oxycodone/acetaminophen and codeine/acetaminophen vs.conventional therapy in elderly women with persistent,moderate to severe osteoarthritis-related pain.
ArchGerontol Geriatr 2009;49:378–382.
|
| 50 |
Friedman BW, Dym AA, Davitt M, et al.
Naproxenwith cyclobenzaprine, oxycodone/acetaminophen, orplacebo for treating acute low back pain: a randomizedclinical trial.
JAMA 2015;314:1572–1580.
|
| 51 |
Conaghan PG, O’Brien CM, Wilson M, Schofield JP.
Transdermal buprenorphine plus oral paracetamol vs anoral codeine-paracetamol combination for osteoarthritisof hip and/or knee: a randomised trial.
Osteoarthritis Cartilage2011;19:930–938.
|
| 52 |
Romanò CL, Romanò D, Lacerenza M.
Antineuropathicand antinociceptive drugs combination in patients withchronic low back pain: a systematic review.
Pain Res Treat2012;2012:154781.
|
| 53 |
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ.
Estimations of worldwide prevalence of chronic hepatitisB virus infection: a systematic review of data publishedbetween 1965 and 2013.
Lancet 2015;386:1546–1555.
|
| 54 |
Marzilawati AR, Ngau YY, Mahadeva S.
Low rates ofhepatotoxicity among Asian patients with paracetamoloverdose: a review of 1024 cases.
BMC Pharmacol Toxicol2012;13:8.
|
| 55 |
The Committee for the Guidelines for Prescribing OpioidAnalgesics for Chronic Non-cancer Pain of JSPC.
JapanSociety of Pain Clinicians—guidelines for prescribingopioid analgesics for chronic non-cancer pain.
Tokyo:Shinko Trading Company Ltd., Publication Departmentof Medical Books; 2012.
|
| 56 |
Cheung CW, Chan TC, Chen PP, et al.
Opioid therapyfor chronic non-cancer pain: guidelines for Hong Kong.
Hong Kong Med J 2016;22:496–505.
|
| 57 |
Kim ED, Lee JY, Son JS, et al.
Guidelines for prescribingopioids for chronic non-cancer pain in Korea.
Korean JPain 2017;30:18–33.
|
| 58 |
Busse JW, Craigie S, Juurlink DN, et al.
Guideline foropioid therapy and chronic noncancer pain.
CMAJ2017;189:E659–E666.
|
Supplmentary Tables
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image