Abstract
Shivering is a common postoperative complication that occurs after both general and regional anesthesia even in the cases when hypothermia during surgery has been averted. Patients describe it as a highly unpleasant experience, while clinicians are concerned due to its adverse effects such as increased oxygen consumption. In this article, we present a summary of the pathophysiological mechanisms involved in postoperative shivering (POS), risk factors, and inadvertent effects. The major objective of this article was to review the existing literature on the efficiency of various drug interventions as a prophylactic measure against POS. Since α2-adrenergic, opioid, anticholinergic, and serotonergic pathways are thought to play a role in the pathogenesis of POS, a wide variety of drugs has been investigated in this regard. Although the methodological diversity of the study designs and regimens does not support drawing definite conclusions, there is evidence indicating a beneficial effect of dexmedetomidine, ketamine, tramadol, meperidine, dexamethasone, nefopam, granisetron, and ondansetron in the prevention of POS. The purpose of this review is to provide a thorough insight on various drug options and to serve as an aid for clinicians for careful analysis of the advantages and disadvantages of each regimen to decide which regimen will be ideally suited for the medical profile of each patient.
Keywords
drugs, preventing, postoperative shivering
Introduction
Postoperative shivering (POS) is defined as readily detectable fasciculations affecting muscle groups, usually appearing early in the postoperative period.1 Its prevalence ranges between 5–65% after general anesthesia and 33–66% after regional anesthesia.2
Pathophysiology
Shivering is the body’s response to augment heat production commonly perceived as a symptom of hypothermia, although core body temperature does not always seem to correlate with the incidence of shivering after anesthesia. Thus, non-thermoregulatory shivering is believed to be caused by special factors related to surgery, such as stress or pain.3-5
Risk Factors
Risk factors that predispose the patient to hypothermia and shivering include young age,6 male gender,7 low body weight, or poor nutritional status,8 prolonged preoperative fasting,9 an American Society of Anesthesiologists (ASA) risk class higher than I,10 combined general-regional anesthesia and the extent of induced sympathetic blockade,8,10 administration of premedication, volatile anesthetics, and muscle relaxants.11
Furthermore, the type and duration of surgery are the surgery-related risk factors for the development of hypothermia. Endoprosthesis and long-lasting abdominal surgery bear a high risk of consequential hypothermia.6 Likewise, intraoperative use of unwarmed irrigation fluids,12 transfusion of cold red blood cells and the low temperature of the operating room (OR)13 predispose the patient to hypothermia.
Adverse Effects
Patients describe shivering as a highly disturbing experience. However, the foremost concern of clinicians lies in the fact that shivering can increase oxygen consumption dramatically, by up to 700% in cases of severe shivering.14 This imposes significant stress on patients, particularly those with burdened cardiorespiratory reserve. Furthermore, shivering increases intraocular15 and intracranial pressure.16 Hypothermia, which may be the underlying cause of shivering, is associated with a number of adverse events,17,18 but discussion on these extends beyond the scope of this review.
Several studies investigating the precautionary measures for shivering have been conducted. This review aims to investigate the existing literature regarding the efficacy of various drugs used to prevent POS.
Methods
Identification of Trials
Published trials (till the September of 2018) investigating the pharmacological options for the prevention of POS were identified by conducting a search on PubMed and Scopus databases, using the keywords “preventing postoperative shivering” and “preventing shivering after anesthesia.”
Inclusion Criteria
We considered solely prospective randomized clinical trials written in English. The investigated drugs had been administered prophylactically at any time between just before the induction of anesthesia till the end of surgery, as a single dose or by infusion, through any parenteral or enteral route.
Exclusion Criteria
We did not include data from retrospective analyses or studies without randomization, to avoid any potential bias. Also, data from abstracts, case reports, nonsurgical settings, cardiac surgery or any other type of surgery using induced hypothermia, guidelines, or from experimental studies using volunteers or animals were excluded.
Results
Trials Included
Of the 244 articles identified initially, only 38 met the selection criteria for this review and were included in the analysis. The flow diagram is illustrated in Fig. 1.
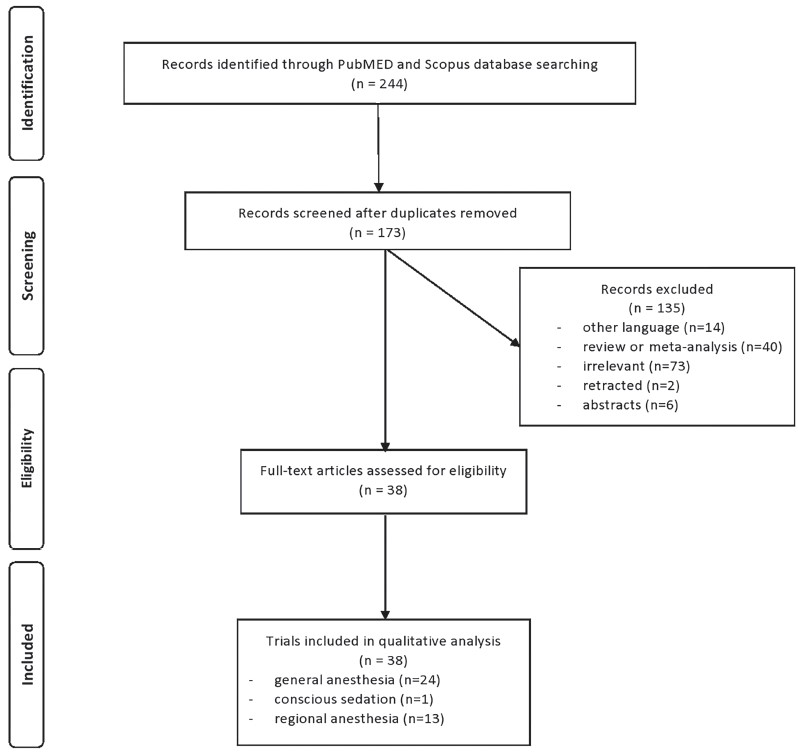
Download full-size image
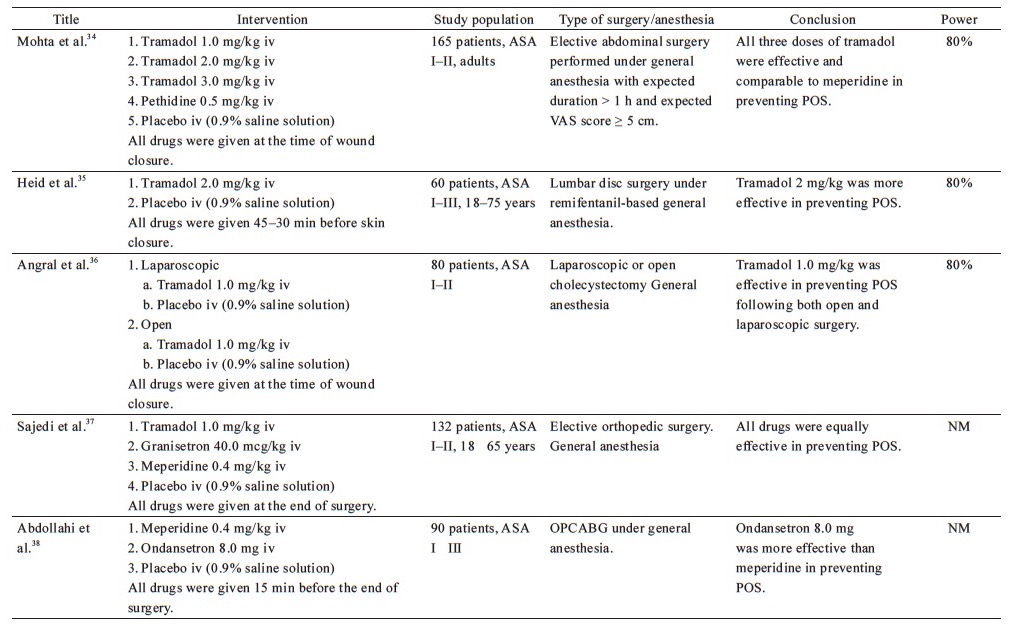
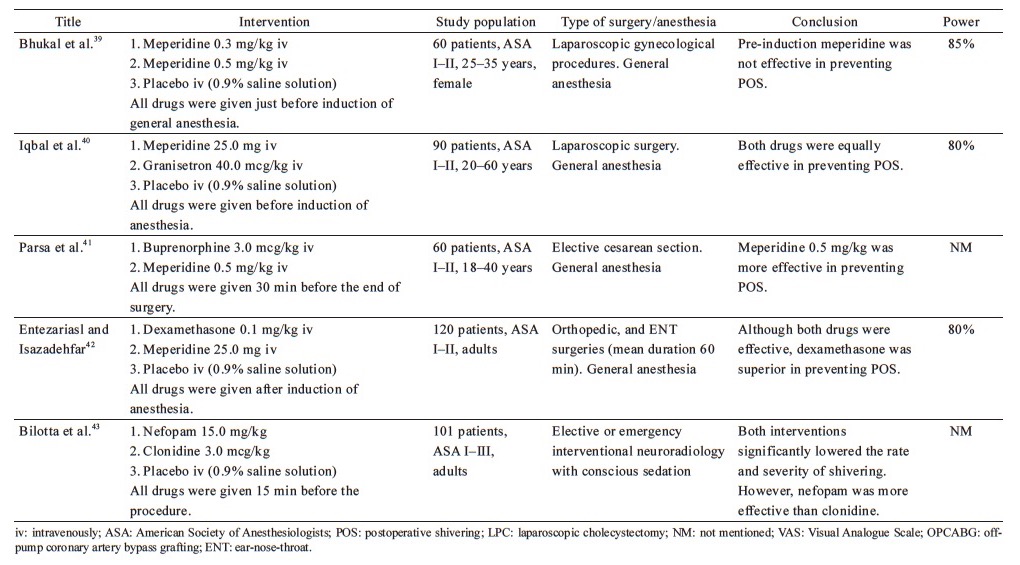
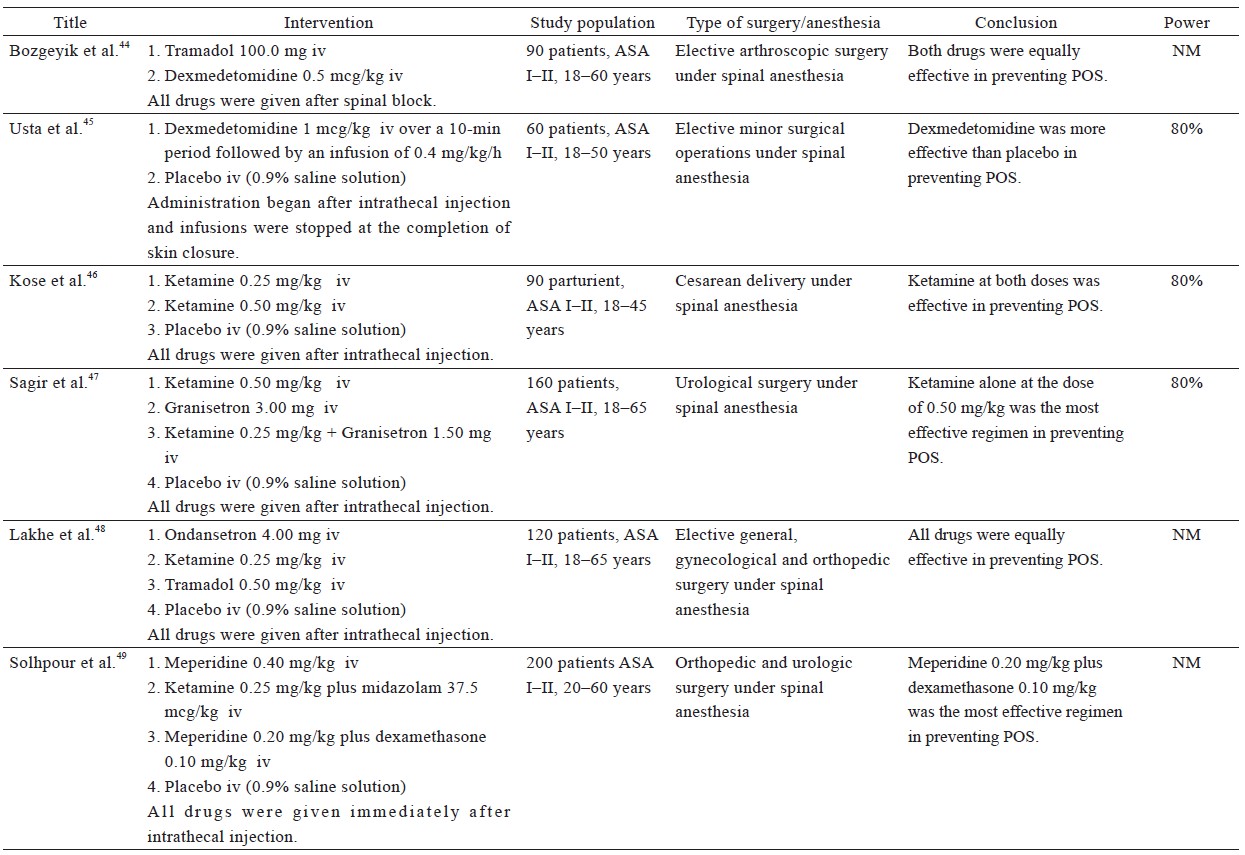
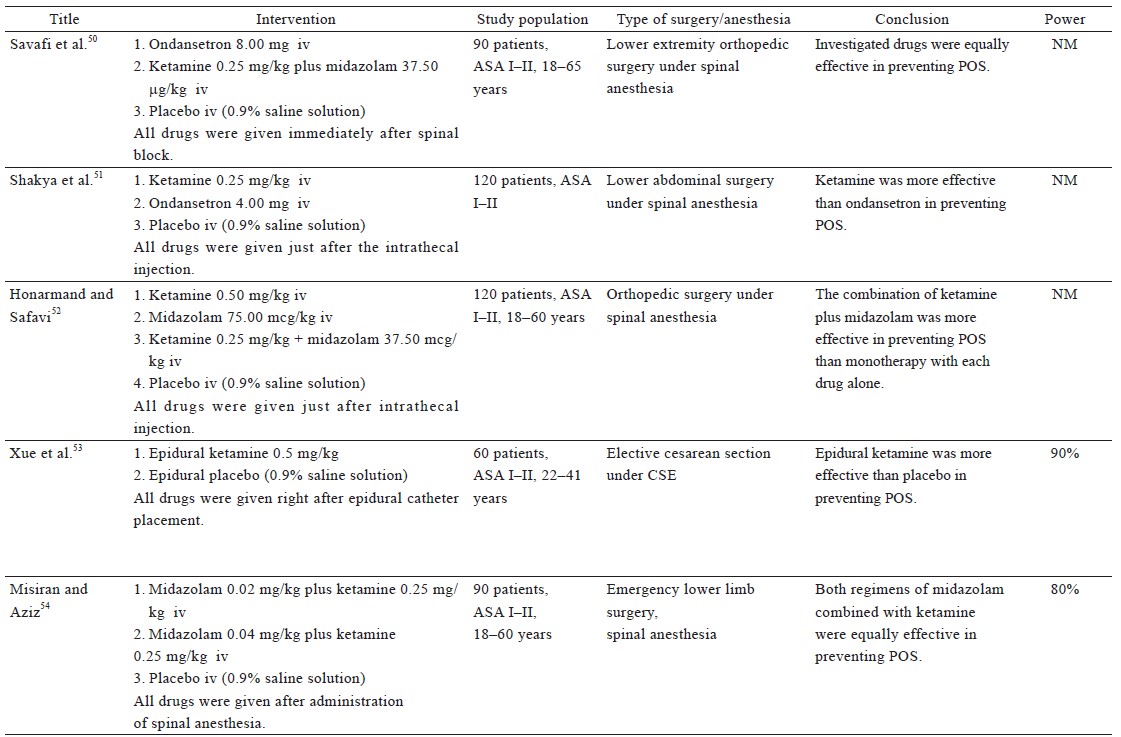
From the 38 included studies, 24 studies had been performed on patients undergoing surgery under general anesthesia and 1 under conscious sedation (Table 1),19-43 and 13 on patients receiving regional anesthesia (Table 2).44-56 Information on patients, type of surgery, investigated drugs (doses, routes, and timing of the administration), power of the study and study findings are presented in these Tables.
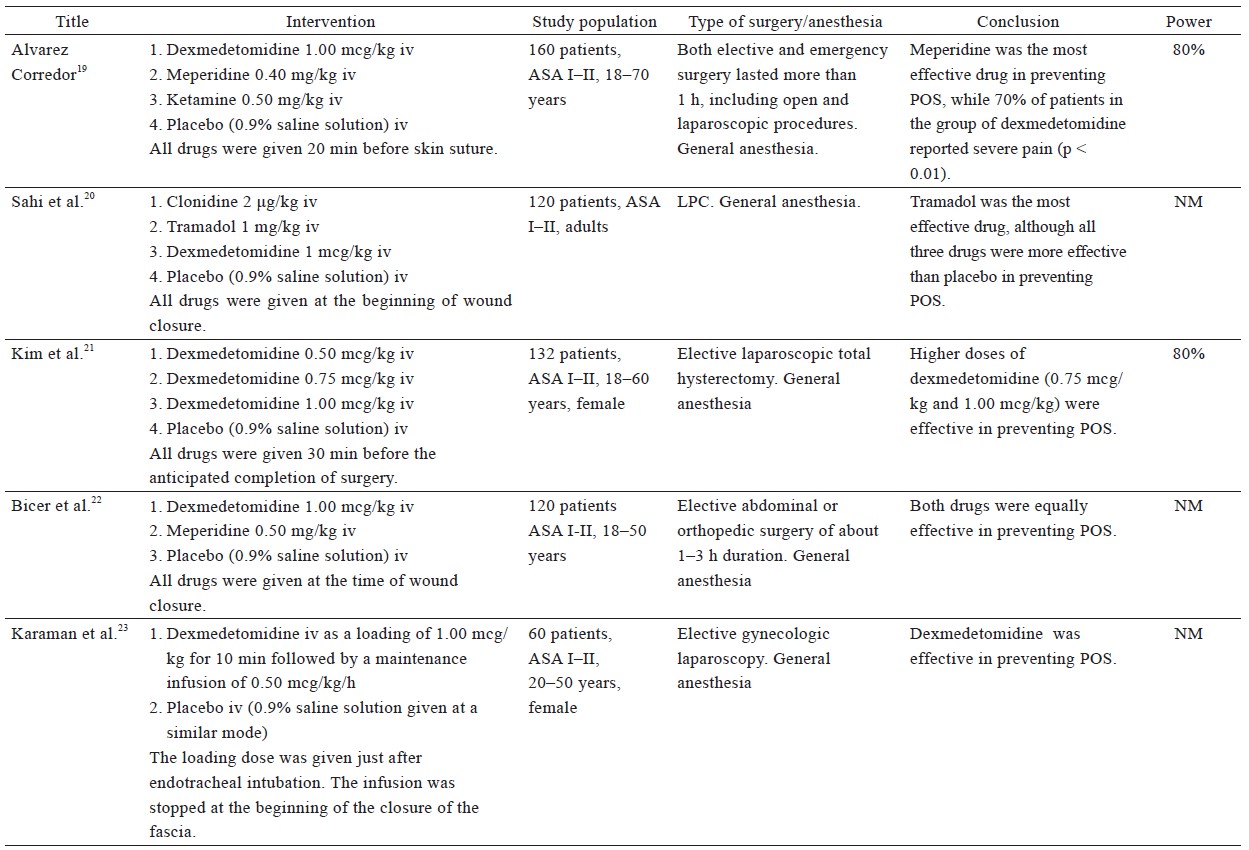
Download full-size image
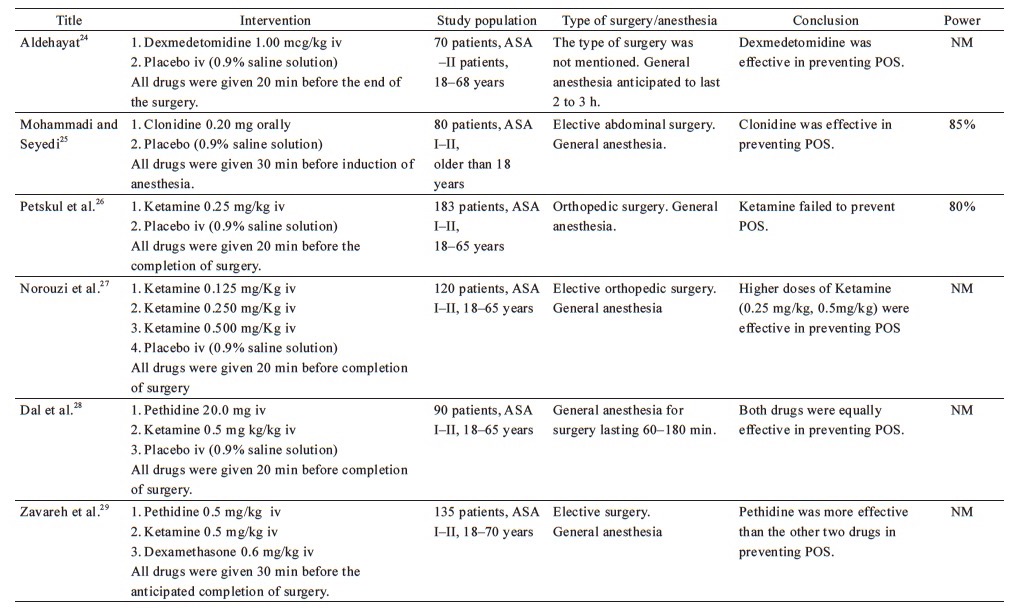
Download full-size image
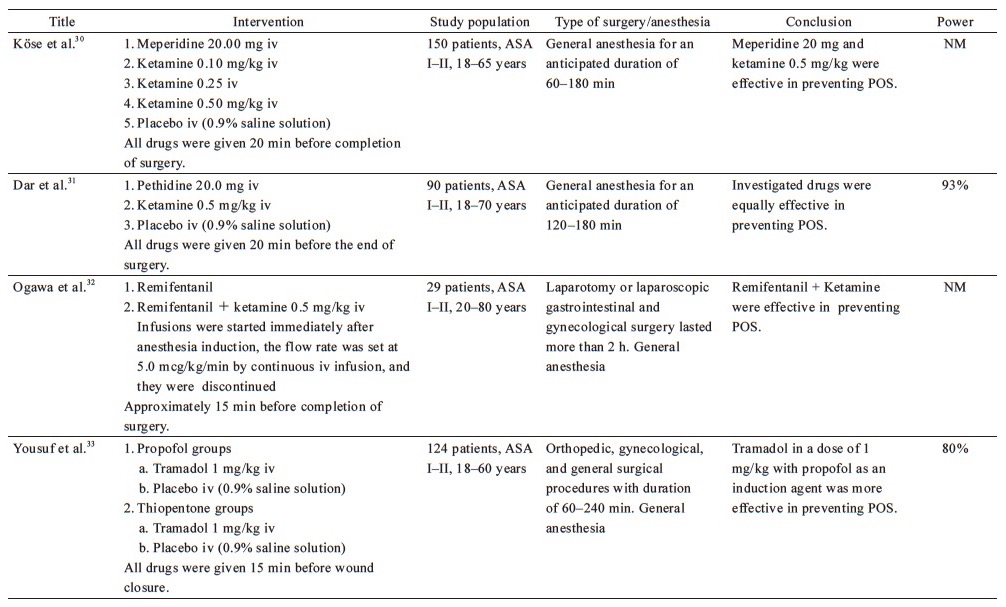
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Since α2-adrenergic, opioid, anticholinergic, and serotonergic pathways are thought to contribute to the pathogenesis of POS, a wide variety of drugs has been investigated regarding their efficacy in its prevention. The route of administration for all the drugs mentioned in this review is mainly intravenous unless stated otherwise.
α2-Adrenergic Agonists
The efficacy of α2-adrenergic agonists in the prevention of POS has been extensively investigated. Dexmedetomidine, with an α1/α2 ratio of 1:1,620, is a highly selective and potent drug of this class, as opposed to clonidine, which is a partial agonist with an α1/α2 ratio of 1:220.57 Due to the selectivity of dexmedetomidine, it is preferred over clonidine in the majority of relevant trials.
General Anesthesia—Τable 1
Our review revealed that clonidine, administered either as oral premedication (0.2 mg)25 or as an intravenous bolus dose (2 mcg/kg) at the end of surgery,20 effectively prevents POS after general anesthesia. Dexmedetomidine (1 mcg/kg) appears to be as efficacious against POS as meperidine (0.5 mg/kg),19,22,24 but is inferior to tramadol (1 mg/kg),20 when administered at the end of surgery. In contrast, Alvarez Corredor reported that 0.4 mg/kg meperidine performed better than 1 mcg/kg dexmedetomidine.19 However, one must also consider that 70% of the patients in the dexmedetomidine group reported severe postoperative pain, while most of the patients in the other groups reported mild to no pain. Since the presence of postoperative pain can facilitate non-thermoregulatory tremors,4 coupled with the fact that the mean duration of anesthesia and surgery, volume of intraoperative IV fluids and blood loss were greater in the dexmedetomidine group, the result may be considerably biased against dexmedetomidine. Kim et al. compared three different dosages of dexmedetomidine (0.50, 0.75, and 1.00 mcg/kg) to placebo and found that dexmedetomidine at 0.75 or 1.00 mcg/kg is an effective prophylactic measure against shivering when administered 30 min before the completion of surgery.21 Furthermore, dexmedetomidine infusion has been successfully used in a placebo-controlled study.23 Sedation, prolonged extubation time, and a tendency of lower mean arterial pressure and heart rate, all of which are dose-dependent adverse effects, were observed in most of the aforementioned studies, without compromising the outcomes of anesthesia.
Regional Anesthesia—Table 2
Only two published studies have investigated the use of dexmedetomidine in the setting of regional anesthesia. The low dose of dexmedetomidine (0.5 mcg/kg), which did not seem to offer significant relief against POS after general anesthesia,21 was found to be similarly effective in preventing POS as 100 mg tramadol, in patients under spinal anesthesia,44 although it was associated with a higher level of sedation. A possible explanation for this discrepancy may be the fact that under general anesthesia, there is a greater suppression of the sympathetic nervous system activity compared to spinal blockade reaching the T8–10 dermatome. Also, continuous infusion of dexmedetomidine was found to be effective in preventing POS resulting from spinal anesthesia.45 In this case, patients are also sedated, but in cases of regional anesthesia, sedation is many times desirable for patients’ comfort.
N-Methyl-D-Aspartate Receptor Antagonists
Research has revealed that N-methyl-D-aspartate (NMDA) receptor antagonists interfere with thermoregulation at various locations within the central nervous system.28
General Anesthesia—Table 1
Ketamine, a non-competitive NMDA receptor antagonist, administered 20 min before the completion of surgery has been investigated at different dosages in the prevention of POS after general anesthesia. Ketamine (0.100 mg/kg or 0.125 mg/kg) does not appear to offer any advantage in the prevention of POS.27,30 Norouzi et al. demonstrated that high doses of ketamine (0.25 mg/kg or 0.50 mg/kg) had similar efficacy in preventing shivering, but the higher dose of ketamine was correlated with an increase in hallucinations and delay in extubation.27 Petskul et al. disagree with this finding, as the dose of 0.25 mg/kg ketamine used in their study failed to prevent POS.26 However, in the aforementioned study, the reported overall incidence of shivering was possibly too low to detect a statistically significant difference.
The efficacy of late administration of ketamine in patients under general anesthesia has also been compared with meperidine, with conflicting results. Ketamine at a dose of 0.5 mg/kg performed better than placebo, but was not as effective as meperidine at doses of 0.4 mg/kg19 or 0.5 mg/kg.29 On the contrary, three studies concluded that 0.5 mg/kg ketamine is as effective as 30 mg meperidine in preventing POS, without significant side effects.28,30,31 This inconsistency might be attributed to the lower dose of meperidine employed in the previous studies.
Ogawa conducted a study to investigate whether shivering associated with remifentanil-based anesthesia could be prevented by the concomitant use of low-dose ketamine infusion, reaching favorable results.32 However, the low number of patients recruited reduces the credibility of the conclusion.
Regional Anesthesia—Table 2
Two studies on adults undergoing elective surgery demonstrated that 0.25 mg/kg ketamine was more effective than placebo in the prevention of POS after spinal anesthesia.48,51 Kose et al. also studied the effects of ketamine on parturients undergoing cesarean section and showed that the prophylactic IV administration of 0.25 mg/kg ketamine was as effective as 0.5 mg/kg ketamine in the prevention of shivering.46 The drug was administered immediately after intrathecal injection and did not exhibit serious maternal or neonatal adverse effects. Xue et al. revealed that prophylactic epidural administration of 0.5 mg/kg ketamine reduced the incidence and severity of shivering in patients undergoing cesarean section under combined spinal-epidural anesthesia.53
The combination of ketamine with midazolam has also been investigated using the rationale that vasoconstriction induced by ketamine is opposed by the effect of midazolam, which impedes tonic thermoregulatory vasoconstriction.58 The study by Savafi et al. yielded positive results regarding the combination of 0.25 mg/kg ketamine plus 37.5 mcg/kg midazolam, for the prevention of POS after spinal anesthesia.50 Another study confirmed this finding and even found the aforementioned combination of ketamine plus midazolam to be more effective than monotherapy using either 0.5 mg/kg ketamine or 75.0 mcg/kg midazolam.52 The same combination was also tested in another study, wherein although it was effective in the prevention of POS, it was found to be inferior to the combination of 0.2 mg/kg meperidine plus 0.1 mg/kg dexamethasone in doing so.49
Misiran and Aziz compared the combination of 0.25 mg/kg ketamine with two different doses of midazolam (0.02 mg/kg vs. 0.04 mg/kg), and concluded that the two regimens were equally effective.54 One peculiarity of this study was that the test group consisted of emergency cases, some of which were trauma cases that were resuscitated in the emergency department prior to surgery. Thus, the pathophysiology of these patients is distinctive, due to which the results of this study cannot be compared with the results of other studies.
A different regimen consisting of ketamine and granisetron (a serotonin 5-HT3 receptor antagonist) has been studied by Sagir et al., who observed that 0.5 mg/kg ketamine was more effective than the combination of a lower dose of ketamine (0.25 mg/kg) plus 1.5 mg granisetron, in the prevention of shivering associated with regional anesthesia.47
Opioids and Tramadol
It is hypothesized that the anti-shivering effect of opioids is associated with their μ-receptor agonist activity.59,60 It seems more probable that the anti-shivering action of meperidine involves its μ- and κ-receptor agonist activity, in addition to its α2-adrenoreceptor agonist properties and anticholinergic properties.14 The fact that the anti-shivering effect of meperidine is inhibited by high-dose naloxone but not by low-dose naloxone indicates that both μ- and κ-receptors play a major role.61
General Anesthesia—Table 1
Most published studies on this topic have investigated the prophylactic effect of meperidine administered at the end of surgery. As mentioned before, meperidine at a dose of 20 mg, administered 20 min before the completion of surgery, is as effective as 0.5 mg/kg ketamine in preventing POS,28,30,31 while higher doses of meperidine (0.4 mg/kg and 0.5 mg/kg) appeared to perform better than 0.5 mg/kg ketamine.19,29 The highest dose of meperidine (0.5 mg/kg) was observed to be as effective as dexmedetomidine 1 mcg/kg.22 Abdollahi et al. compared 0.4 mg/kg meperidine with 8 mg ondansetron in patients undergoing off-pump coronary artery bypass graft who, after the end of surgery, were transferred to intensive care unit (ICU) and remained intubated.38 The shivering scores recorded at the time of ICU admission were in favor of 0.4 mg/kg meperidine and 8 mg ondansetron, compared to placebo.
Three other studies had tested the prophylactic administration of meperidine given before or right after the induction of anesthesia.39,40,42 Entezariasl and Isazadehfar compared the efficacy of 25 mg meperidine with 0.1 mg/kg dexamethasone, both given immediately after the induction of anesthesia. The researchers observed that the incidence of POS was 10% in the dexamethasone group, 37.5% in the meperidine group, and 47.5% in the control group.42 The findings of Iqbal et al. also support the prophylactic administration of meperidine before the induction of anesthesia.40 In contrast, Bhukal et al. have disputed the pre-induction efficacy of meperidine.39 This inconsistency may be attributed to the fact that they studied only female patients, and intravenous fluids administered were heated to 37°C.39
The study by Parsa et al. is unique in that it compared the efficacy of buprenorphine to meperidine in a group of parturients undergoing cesarean section under general anesthesia.41 The researchers valued buprenorphine for being a partial μ-receptor agonist and a betaine derivative that is attached to κ- and σ-receptors, and observed that although both drugs decreased postoperative pain equally, meperidine performed better as an anti-shivering agent.41
Tramadol acts centrally on μ-opioid receptors, inhibits the reuptake of 5-hydroxytryptamine and norepinephrine, and facilitates the release of 5-hydroxytryptamine. In 2017, a meta-analysis by Li et al. showed that tramadol was an effective cure against POS by significantly reducing the severity of shivering and the need for a rescue agent.62 No adverse events were associated with its use, even when doses as high as 3 mg/kg were employed. In any case, authors of this meta-analysis recommended the dose of 1 mg/kg, in order to avoid the possibility of adverse effects.
Mohta et al. compared different doses of tramadol (1, 2, and 3 mg/kg) for their anti-shivering and analgesic effects.34 Tramadol at a dose of 2 mg/kg, administered at the time of wound closure, appeared to have the best anti-shivering and analgesic effect, without any undesirable adverse effects. It is worth noting that all patients received prophylaxis for postoperative nausea and vomiting (PONV) using 10 mg metoclopramide. The three groups of tramadol were found to be as efficient as 0.5 mg/kg meperidine in terms of POS prevention. Findings of few studies confirm that 1 mg/kg tramadol administered at the end of surgery prevents POS after general anesthesia33 and is equally effective to 0.4 mg/kg meperidine.37 Furthermore, 1 mg/kg tramadol is more efficient than α2-agonists.20 Notably, the higher dose of 2 mg/kg was even found to be effective in remifentanil-isoflurane-based general anesthesia, which is associated with a particularly high incidence of POS.35
Angral et al. studied the usage of 1 mg/kg tramadol, administered at the time of wound closure, in open and laparoscopic cholecystectomy.36 The incidence of POS was similarly low in the treatment groups, but significantly higher in the control groups.36
Regional Anesthesia—Table 2
Even though meperidine is highly valued as an anti-shivering agent, our search revealed only one study that had employed meperidine as anti-shivering prophylaxis for regional anesthesia. According to this study, a combination of 0.2 mg/kg meperidine plus 0.1 mg/kg dexamethasone was more effective than 0.4 mg/kg meperidine alone or combination of 0.25 mg/kg ketamine plus 37.5 mcg/kg midazolam, in the prevention of shivering resulting from spinal anesthesia.49
Tramadol at doses of 0.5 mg/kg has been found to be as effective in preventing POS as 0.5 mg/kg meperidine, with negligible side effects.48 Bozgeyik et al. claim that 100 mg tramadol acts as effectively as 0.5 mcg/kg dexmedetomidine.44
Serotonin 5-HT3 Receptor Antagonists
The mechanism of action of serotonin 5-HT3 receptor antagonists could be related to the inhibition of serotonin reuptake on the preoptic anterior hypothalamic region. The 5-HT3 receptors may also influence both heat production and heat loss pathways.
General Anesthesia—Table 1
Iqbal et al. have shown that the prophylactic use of 40 mcg/kg granisetron is as effective as 25 mg meperidine in preventing POS.40 It is worth mentioning that this dose was found to be even equally effective to a higher dose of meperidine (0.4 mg/kg) or to tramadol (1 mg/kg) without prolonging the time of emergence from anesthesia or affecting the respiratory or cardiovascular system.37 Likewise, Abdollahi et al. have compared the efficacy of ondansetron (8 mg) to meperidine (0.4 mg/kg) on a population more prone to cardiovascular side effects, and observed no significant difference in the incidence of side effects such as bradycardia, convulsion, rash, and myoclonus between groups.38
Regional Anesthesia—Table 2
Serotonin 5-HT3 receptor antagonists have been studied for the prophylaxis of POS-related to regional anesthesia and compared to various regimens. Sagir et al. commented that 3 mg granisetron alone or a combination of 0.25 mg/kg ketamine plus 1.5 mg granisetron were inferior to 0.5 mg/kg ketamine in preventing shivering related to regional anesthesia.47 Savafi et al. reported that although 8 mg ondansetron was effective in controlling POS, a combination of 0.25 mg/kg ketamine plus 37.5 mcg/kg midazolam performed better.50 Ondansetron at a lower dose (4 mg) still appears to be effective but is inferior to 0.25 mg/kg ketamine.51 On the contrary, Lakhe et al. showed that 4 mg ondansetron is as effective as 0.25 mg/kg ketamine or 0.5 mg/kg tramadol.48
Dexamethasone
The available data on dexamethasone efficacy are rare. It has been proposed that dexamethasone decreases the gradient between core and skin temperature and inhibits the release of vasoconstrictors and pyrogenic cytokines released during surgery, through its action on the immune system.63
General Anesthesia—Table 1
As mentioned earlier, Entezariasl and Isazadehfar showed that dexamethasone surpassed meperidine’s efficacy in preventing POS, when administered after induction of anesthesia.42
Regional Anesthesia—Table 2
Solhpour et al. observed that the combination of meperidine and dexamethasone is superior to meperidine alone, in preventing POS in patients subjected to spinal anesthesia.49
Nefopam
Nefopam is a non-opioid, non-steroidal, centrally acting analgesic drug that increases the activity of serotonin, norepinephrine, and dopamine. Furthermore, it acts on the sodium and calcium channels, inhibiting the release of glutamate64 and it seems to have a promising role in the prevention of POS.
Conscious Sedation—Table 1
We opted for including in our review a randomized control trial that compared the efficacy of nefopam and clonidine on patients undergoing interventional neuroradiological procedures under conscious sedation with midazolam infusion. In such cases, prevention of shivering is essential, as any movement can cause technical difficulties or even complications, such as vessel perforation.43 Authors concluded that although both drugs are effective in preventing POS, nefopam is more potent than clonidine. In addition, patients receiving nefopam were more hemodynamically stable than patients in clonidine and placebo groups.
Regional Anesthesia—Table 2
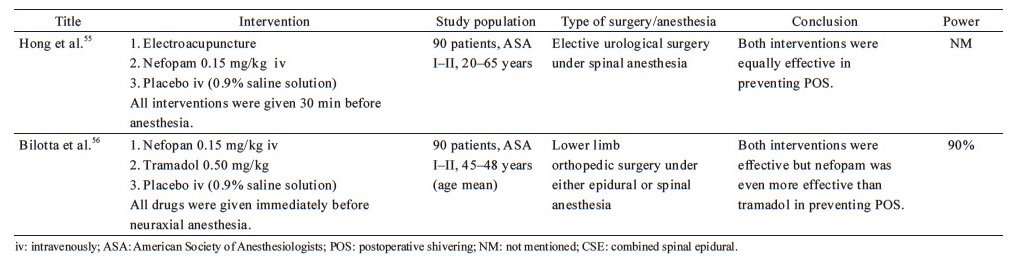
According to Hong et al., 0.15 mg/kg nefopam was efficient in preventing POS after spinal anesthesia, but nausea and injection pain were observed.55 Nefopam even surpassed tramadol’s efficacy in lowering the rate and severity of intraoperative shivering in patients undergoing neuraxial anesthesia for orthopedic surgery, according to Bilotta et al.56 However, the dose of tramadol (0.5 mg/kg) studied was possible too low to evaluate its full effect in preventing shivering.
Conclusion
Although the published studies on this topic are greatly heterogeneous in factors such as temperature of the OR, type and duration of surgery, anesthetic drugs used, intraoperative fluid replacement, use of active warming measures, as well as the scales used to evaluate shivering, there is evidence that some drugs can effectively prevent POS. However, dose-response studies are required to determine the most appropriate dosing regimen of each drug for the optimal prevention of POS. Studies of cost-effectiveness are also essential.
Regarding α2-agonists, intravenous dexmedetomidine was found to be promising. It appears to have a dose-dependent preventive effect on patients subjected to general anesthesia. Notably, meperidine and tramadol surpass its efficacy in most studies, and the expected side effects associated with α2-agonists, including bradycardia, sedation in the immediate postoperative period, and prolonged extubation time, are observed. Although limited data are available regarding regional anesthesia, it appears that the continuous infusion of dexmedetomidine, or even the low dose of 0.5 mcg/kg, is effective in preventing POS.
Regarding NMDA antagonists, 0.25 mg/kg ketamine has questionable efficacy in preventing POS after general anesthesia, while lower doses do not seem to confer any advantage at all. Ketamine at a dose of 0.5 mg/kg performs better than placebo and possibly, is even equally effective to meperidine. Despite the number of studies concerning regional anesthesia, the diverse regimens used in each study makes it infeasible to draw safe conclusions. In general, the rationale for the use of ketamine in the setting of POS is that it could serve as an alternative drug for patients with bradycardia, hypotension, respiratory depression, nausea, vomiting, or allergic reactions to pethidine. Still, adverse effects (hallucination, nystagmus, sedation, etc.) should always be borne in mind.
As far as opioids are concerned, 0.4–0.5 mg/kg meperidine is effective in the prevention of POS after general anesthesia, when administered at the end of surgery, and possibly, even when administered at the beginning. It appears to be effective in patients with different ASA classifications and undergoing different procedures. There are not enough data available to comment on the efficacy of meperidine after regional anesthesia.
As for tramadol, the dose of 1–2 mg/kg appeared to be effective as anti-shivering prophylaxis after general anesthesia, without adverse effects including PONV. However, most of the studies involved patients that had been administered metoclopramide in advance. Tramadol was found to be effective as a preventive measure for shivering under regional anesthesia, but the ideal dose is not established yet.
Additionally, the use of serotonin 5-HT3 receptor antagonists, such as ondansetron or granisetron, seems promising as a preventive measure against POS. The main advantage of this class of drugs is that they prevent PONV and do not prolong the emergence time from anesthesia or significantly affect the respiratory or cardiovascular system.
Finally, as for dexamethasone, data are limited on reaching definite conclusions. On the contrary, nefopam seems to play an important role in the prevention of POS, since it has been found to surpass tramadol and clonidine’s efficacy and to possess a favorable hemodynamic profile.
In conclusion, even though an association between the occurrence of shivering and an increase in cardiac morbidity has not yet been established, POS should be avoided because it raises the oxygen demand. Consequently, as with all other preventive measures, the prophylactic administration of the aforementioned drugs should also be considered in patients at a high risk of developing POS. However, clinicians should carefully weigh the advantages of preventing shivering against the risk of inadvertent drug reactions. Thus, a personalized approach should be applied and the regimen best suited to the medical profile of each patient should be selected. In this regard, this review contributes to a rational framework for daily clinical practice.
Conflicts of Interest
None.
References
| 1 |
Buggy DJ, Crossley AWA.
Thermoregulation, mild perioperative hypothermia and post-anaesthetic shivering.
Br J Anaesth 2000;84:615–628.
|
| 2 | |
| 3 |
Sessler DI, Rubinstein EH, Moayeri A.
Physiologic responses to mild perianesthetic hypothermia in humans.
Anesthesiology 1991;75:594–610.
|
| 4 |
Horn EP, Schroeder F, Wilhelm S, et al.
Postoperative pain facilitates nonthermoregulatory tremor.
Anesthesiology 1999;91:979–984.
|
| 5 |
Panzer O, Ghazanfari N, Sessler DI, et al.
Shivering and shivering-like tremor during labor with and without epidural analgesia.
Anesthesiology 1999;90:1609–1616.
|
| 6 |
Eberhart LH, Döderlein F, Eisenhardt G, et al.
Independent risk factors for postoperative shivering.
Anesth Analg 2005;101:1849–1857.
|
| 7 |
Crossley AW.
Six months of shivering in a district general hospital.
Anesthesia. 1992;47:845–848.
|
| 8 |
Kongsayreepong S, Chaibundit C, Chadpaibool J, et al.
Predictor of core hypothermia and the surgical intensive care unit.
Anesth Analg 2003;96:826–833.
|
| 9 |
Bernthal EM.
Inadvertent hypothermia prevention: the anaesthetic nurses’ role.
Br J Nurs 1999;8:17–25.
|
| 10 |
Torossian A, Bräuer A, Höcker J, Bein B, Wulf H, Horn EP.
Preventing inadvertent perioperative hypothermia.
Dtsch Arztebl Int 2015;112:166–172.
|
| 11 | |
| 12 |
De Witte J, Sessler DI.
Perioperative shivering: physiology and pharmacology.
Anesthesiology 2002;96:467–484.
|
| 13 |
El-Gamal N, Elkassabany N, Frank SM, et al.
Age-related thermoregulatory differences in a warm operating room environment (approximately 26 degrees C).
Anesth Analg 2000;90:694–698.
|
| 14 |
Alfonsi P.
Postanaesthetic shivering. Epidemiology, pathophysiology and approaches.
Minerva Anestesiol 2003;69:438–442.
|
| 15 |
Mahajan RP, Grover VK, Sharma SL, Singh H.
Intraocular pressure changes during muscular hyperactivity after general anesthesia.
Anesthesiology 1987;66:419–421.
|
| 16 |
Rosa G, Pinto G, Orsi P, et al.
Control of post anaesthetic shivering with nefopam hydrochloride in mildly hypothermic patients after neurosurgery.
Acta Anaesthesiol Scand 1995;39:90–95.
|
| 17 |
Sessler DI, Olofsson CI, Rubinstein EH.
The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia.
Anesthesiology 1988;69:357–364.
|
| 18 |
Sheffield CW, Sessler DI, Hopf HW, et al.
Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension.
Wound Repair Regen 1996;4:339–345.
|
| 19 |
Alvarez Corredor FA.
Comparison of the effectiveness of dexmedetomidine, meperidine and ketamine in the prevention of postoperative shivering.
Rev Esp Anestesiol Reanim 2016;63:505–512.
|
| 20 |
Sahi S, Singh MR, Katyal S.
Comparative efficacy of intravenous dexmedetomidine, clonidine, and tramadol in postanesthesia shivering.
J Anaesthesiol Clin Pharmacol 2016;32:240–244.
|
| 21 |
Kim YS, Kim YI, Seo KH, Kang HR.
Optimal dose of prophylactic dexmedetomidine for preventing postoperative shivering.
Int J Med Sci 2013;10:1327–1332.
|
| 22 |
Bicer C, Esmaoglu A, Akin A, Boyaci A.
Dexmedetomidine and meperidine prevent postanaesthetic shivering.
Eur J Anaesthesiol 2006;23:149–153.
|
| 23 |
Karaman S, Günüşen I, Ceylan MA, et al.
Dexmedetomidine infusion prevents postoperative shivering in patients undergoing gynecologic laparoscopic surgery.
Turk J Med Sci 2013;43:232–237.
|
| 24 |
Aldehayat G.
Intraoperative dexmedetomidine administration at the end of surgery prevents post anesthetic shivering.
Rawal Med J 2011;36:274–276.
|
| 25 |
Mohammadi SS, Seyedi M.
Effects of oral clonidine in preventing postoperative shivering after general anesthesia.
Int J Pharmacol 2007;3:441–443.
|
| 26 |
Petskul S, Kitsiripant C, Rujirojindakul P, Chantarokorn A, Jullabunyasit A, Thinchana S.
Prophylactic low-dose ketamine to prevent post anesthetic shivering in orthopedic surgery: a randomized-controlled study.
J Med Assoc Thai 2016;99:400–405.
|
| 27 |
Norouzi M, Doroodian MR, Salajegheh S.
Optimum dose of ketamine for prevention of postanesthetic shivering; a randomized double-blind placebo-controlled clinical trial.
Acta Anaesthesiol Belg 2011;62:33–36.
|
| 28 |
Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U.
Efficacy of prophylactic ketamine in preventing postoperative shivering.
Br J Anaesth 2005;95:189–192.
|
| 29 |
Zavareh SMHT, Morovati L, Koushki AM.
A comparative study on the prophylactic effects of ketamine, dexamethasone, and pethidine in preventing postoperative shivering.
J Res Med Sci 2012;2:S175–S181
|
| 30 |
Köse EA, Honca M, Akinci SB, Dal D, Aypar Ü.
Efficacy of prophylactic ketamine in preventing postoperative shivering.
J Clini Anal Med 2012;3:182–185.
|
| 31 |
Dar AM, Qazi SM, Sidiq S.
A placebo-controlled comparison of ketamine with pethidine for the prevention of postoperative shivering.
S Afr J Anaesth Analg 2012;18:340–343.
|
| 32 |
Ogawa S, Okutani R, Suehiro K, Shigemoto T.
The efficacy of ketamine for the prevention of remifentanil-induced postoperative shivering.
Anesth Resus 2008;44:77–80.
|
| 33 |
Yousuf B, Samad K, Ullah H, Hoda MQ.
Efficacy of tramadol in preventing postoperative shivering using thiopentone or propofol as induction agent: a randomized controlled trial.
J Anaesthesiol Clin Pharmacol 2013;29:521–525.
|
| 34 |
Mohta M, Kumari N, Tyagi A, Sethi AK, Agarwal D, Singh M.
Tramadol for prevention of postanaesthetic shivering: a randomised double-blind comparison with pethidine.
Anaesthesia 2009;64:141–146.
|
| 35 |
Heid F, Grimm U, Roth W, Piepho T, Kerz T, Jage J.
Intraoperative tramadol reduces shivering but not pain after remifentanil-isoflurane general anaesthesia. A placebo-controlled, double-blind trial.
Eur J Anaesthesiol 2008;25:468–472.
|
| 36 |
Angral R, Wani AA, Kapoor BB.
Tramadol and postoperative shivering in patients undergoing open and laparoscopic cholecystectomy under general anaesthesia.
S Afr J Anaesth Analg 2012;18:111–114.
|
| 37 |
Sajedi P, Yaraghi A, Moseli HA.
Efficacy of granisetron in preventing postanesthetic shivering.
Acta Anaesthesiol Taiwan 2008;46:166−170.
|
| 38 |
Abdollahi MH, Forouzannia SK, Bagherinasab M, et al.
The effect of ondansetron and meperedin on preventing shivering after off-pump coronary artery bypass graft.
Acta Med Iran 2012;50:395–398.
|
| 39 |
Bhukal I, Solanki SL, Kumar S, Jain A.
Pre-induction low dose pethidine does not decrease incidence of postoperative shivering in laparoscopic gynecological surgeries.
J Anaesth Clin Pharmacol 2011;27:349–353.
|
| 40 |
Iqbal A, Ahmed A, Rudra A, et al.
Prophylactic granisetron vs pethidine for the prevention of postoperative shivering: a randomized control trial.
Indian J Anaesth 2009;53:330–334.
|
| 41 |
Parsa T, Dabir S, Radpay B.
Efficacy of pethidine and buprenorphine for prevention and treatment of postanesthetic shivering.
Tanaffos 2007;6:54–58.
|
| 42 |
Entezariasl M, Isazadehfar K.
Dexamethasone for prevention of postoperative shivering: a randomized double-blind comparison with pethidine.
Int J Prev Med 2013;4:818–824.
|
| 43 |
Bilotta F, Ferri F, Giovannini F, Pinto G, Rosa G.
Nefopam or clonidine in the pharmacologic prevention of shivering in patients undergoing conscious sedation for interventional neuroradiology.
Anaesthesia 2005;60:124–128.
|
| 44 |
Bozgeyik S, Mizrak A, Kılıç E, Yendi F, Ugur BK.
The effects of preemptive tramadol and dexmedetomidine on shivering during arthroscopy.
Saudi J Anaesth 2014;8:238–243.
|
| 45 |
Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A.
Dexmedetomidine for the prevention of shivering during spinal anesthesia.
Clinics (Sao Paulo) 2011;66:1187–1191.
|
| 46 |
Kose EA, Honca M, Dal D, Akinci SB, Aypar U.
Prophylactic ketamine to prevent shivering in parturients undergoing Cesarean delivery during spinal anesthesia.
J Clin Anesth 2013;25:275–280.
|
| 47 |
Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O.
Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron.
Acta Anaesthesiol Scand 2007;51:44–49.
|
| 48 |
Lakhe G, Adhikari KM, Khatri K, Maharjan A, Bajracharya A, Khanal H.
Prevention of shivering during spinal anesthesia : comparison between tramadol, ketamine and ondansetron.
JNMA J Nepal Med Assoc 2017;56:395–400.
|
| 49 |
Solhpour A, Jafari A, Hashemi M, et al.
A comparison of prophylactic use of meperidine, meperidine plus dexamethasone, and ketamine plus midazolam for preventing of shivering during spinal anesthesia: a randomized, double-blind, placebo-controlled study.
J Clin Anesth 2016;34:128–135.
|
| 50 |
Savafi M, Honarmand A, Mohammadsadeqie S.
Prophylactic use of intravenous ondansetron versus ketamine-midazolam combination for prevention of shivering during spinal anesthesia: a randomized double-blind placebo-controlled trial.
Adv Biomed Res 2015;4:207.
|
| 51 |
Shakya B, Chaturvedi A, Sah BP.
Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia.
J Anaesthesiol Clin Pharmacol 2010;26:465–469.
|
| 52 |
Honarmand A, Safavi MR.
Comparison of prophylactic use of midazolam, ketamine, and ketamine plus midazolam for prevention of shivering during regional anaesthesia: a randomized double-blind placebo controlled trial.
Br J Anaesth 2008;101:557–562.
|
| 53 |
Xue X, Lv Y, Zhao Y, Leng Y, Zhang Y.
Efficacy of prophylactic epidural ketamine for reducing shivering in patients undergoing caesarean section with combined spinal-epidural anesthesia.
Biomed Rep 2018;8:485–490.
|
| 54 |
Misiran K, Aziz MZ.
Effectiveness of low-dose midazolam plus ketamine in the prevention of shivering during spinal anaesthesia for emergency lower limb surgery.
S Afr J Anaesth Analg 2013;19:164–170.
|
| 55 |
Hong JH, Kim SJ, Hwang MS.
Comparison of effect of electroacupuncture and nefopam for prevention of postanesthetic shivering in patients undergoing urologic operation under spinal anesthesia.
Korean J Anesthesiol 2016;69:579–586.
|
| 56 |
Billotta F, Pietropaoli P, Sanitá R, Liberatori G, Rosa G.
Nefopam and tramadol for the prevention of shivering during neuraxial anesthesia.
Reg Anesth Pain Med 2002;27:380–384.
|
| 57 |
Masuki S, Dinenno FA, Joyner MJ, Eisenach JH.
Selective alpha2-adrenergic properties of dexmedetomidine over clonidine in the human forearm.
J Appl Physiol 2005;99:587–592.
|
| 58 |
Grover VK, Mahajan R, Yaddanapudi LN, Sudarshana HG, Gill KD.
Efficacy of midazolam in preventing postoperative shivering.
Int J Clin Pharmacol Ther 2002;40:534–536.
|
| 59 |
Alfonsi P, Sessler DI, Du Manoir B, Levron JC, Le Moing JP, Chauvin M.
The effects of meperidine and sufentanil on the shivering threshold in postoperative patients.
Anesthesiology 1998;89:43–48.
|
| 60 |
Kurz A, Ikeda T, Sessler DI, et al.
Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold.
Anesthesiology 1997;86:1046–1054.
|
| 61 |
Wrench IJ, Cavill G, Ward JE, Crossley AW.
Comparison between alfentanil, pethidine and placebo in treatment of post-anaesthetic shivering.
Br J Anaesth 1997;79:541–542.
|
| 62 |
Li S, Li P, Lin X.
Efficacy of the prophylactic administration of tramadol against postoperative shivering: a meta-analysis of randomized controlled trials.
Minerva Anestesiol 2017;83:79–87.
|
| 63 |
Yared JP, Starr NJ, Hoffmann-Hogg L, et al.
Dexamethasone decreases the incidence of shivering after cardiac surgery: a randomized, double-blind, placebo-controlled study.
Anesth Analg 1998;87:795–799.
|
| 64 |
Girard P, Chauvin M, Verleye M.
Nefopam analgesia and its role in multimodal analgesia: a review of preclinical and clinical studies.
Clin Exp Pharmacol Physiol 2016;43:3–12.
|