To the Editor,
Peripheral nerve blocks are used effectively as intraoperative and postoperative analgesia. Nerve blocks are often performed in conscious patients to minimize neurological complications, but the procedures may be preferable under general anesthesia in some patients, such as children, uncooperative patients, and patients who strongly desire to receive the block under general anesthesia.1 When visualization of the nerve and/or the needle is difficult with ultrasound alone, combined ultrasound and nerve stimulation guidance (“dual-guidance”) is helpful for peripheral nerve blocks.2 However, muscle responses to nerve stimulation cannot be obtained immediately after the induction of general anesthesia and administration of muscle relaxants. Muscle relaxants are not needed for general anesthesia using a supraglottic airway device, but in some cases anesthesiologists may prefer tracheal intubation under muscle relaxation. Currently, there is no established standard method for recovery of neuromuscular function for nerve stimulation with a peripheral nerve block. We examined the hypothesis that administration of sugammadex 2 mg/kg ten minutes after injection of rocuronium 0.6 mg/kg would facilitate early recovery of neuromuscular function, enabling us to obtain adequate responses to nerve stimulation during peripheral nerve blocks under general anesthesia.
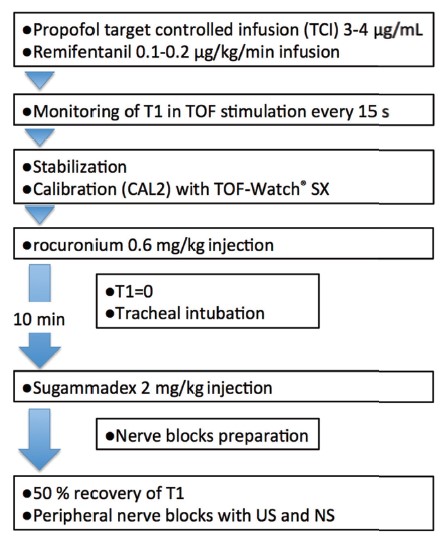
This study was registered with University Hospital Medical Information Network (UMIN) Clinical Trials (UMIN000027916) and approved by the institutional ethics committee (protocol number. H29-1); 27 patients were enrolled. Data were obtained from 25 adult patients who desired to receive general anesthesia for upper and/or lower limb surgery and to receive peripheral nerve block for pain control during general anesthesia. Patients with neuromuscular disease, neuropathy, or any allergies to anesthetics were excluded. Written informed consent was obtained from all patients. Total intravenous anesthesia with propofol and remifentanil was used in all patients (Figure 1). Neuromuscular monitoring was continuously recorded using a TOF-Watch® SX (Organon Ireland Ltd., Swords, Ireland) with the adductor pollicis muscle as recommended.3 After obtaining stable responses with nerve stimulation, rocuronium 0.6 mg/kg was administered, and tracheal intubation was performed. Sugammadex 2 mg/kg was administered to all patients after ten minutes of rocuronium injection. After obtaining 50% recovery of the fi rst twitch (T1) in train-of-four (TOF) stimulation, brachial plexus, femoral and/or popliteal sciatic nerve blocks were performed for each surgery using combined ultrasound and nerve stimulation technique. A total of 15–20 mL of ropivacaine 0.375% was used for each block.
Download full-size image
Abbreviations: NS, nerve stimulation; T1, fi rst twitch height; TOF, train-of-four stimulation; US, ultrasound.
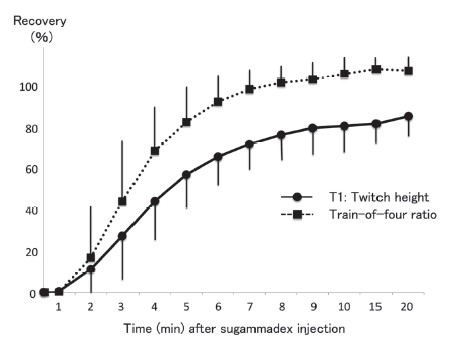
The surgeries included upper arm (n = 7), elbow (n = 1), wrist (n = 9), hip (n = 3), knee (n = 5), foot (n = 1). One patient received wrist and hip surgeries on the same day. A total of 32 nerve blocks were performed in 25 patients as seven patients received two blocks. Nerve blocks inclu brachial plexus block (supraclavicular, n = 16; axillary, n = 1), femoral nerve block (n = 9) and sciatic nerve block (n = 6). Nerve stimulation guidance was successful in 31 of 32 blocks (96.9%). Muscle response to nerve stimulation with a current of 0.5–1.0 mA was subjectively judged to be sufficient in these patients. Muscle response was judged as poor in one patient because the response was weak and a stimulating current of more than 1.0 mA was required. All blocks were judged to be successful by complete loss of postoperative pain. The mean time from sugammadex injection to the recovery of T1 to above 50% was 4.6 (SD, 1.4) minutes. The recovery pattern of T1 and the TOF ratio for 20 minutes after sugammadex administration is shown in Figure 2. During positioning for surgery, coughing and/or movement were noted as adverse events in 18 patients. They were almost slight and treated with an additional injection of propofol, fentanyl, and/or rocuronium. Residual neuromuscular block (TOF ratio of < 90%) at the end of surgery, recurarization, and neurological complications with nerve blocks were not noted in any patients.
Download full-size image
Values are expressed as mean and SD. Peripheral nerve blocks were performed after 50% recovery of T1 response was obtained.
Nerve stimulation with ultrasound guidance for peripheral nerve blocks could be performed by using sugammadex in the early period after the induction of general anesthesia. The time interval between the administration of rocuronium and sugammadex may affect the recovery of rocuronium by sugammadex. In the previous study of neural monitoring,4 the time interval was approximately 16 minutes (sugammadex injected at the time of skin incision). Therefore, we attempted a shorter interval (10 minutes). The recovery pattern of T1 and TOF was similar between the two studies (Figure 2). The best interval for rapid reversal is unclear. Within the ten minutes interval, tracheal intubation can be performed even in difficult cases. The dose of sugammadex may also affect recovery. Higher doses allow faster recovery from neuromuscular blockade.5 However, we did not use more than 2 mg/kg due to costs consideration. The 50% recovery of T1 was used as the time point to perform nerve block with nerve stimulation, which corresponded to 70–80% recovery of TOF in the previous4 and current studies. Thus, muscle responses to nerve stimulation may be suffi cient at this point. We selected the generally recommended stimulating current of 0.5–1.0 mA,2 which functioned well in most of our patients. However, it should be cautioned that muscle responses could have been underestimated during the recovery period from muscle relaxants. To prevent nerve injury by intraneural injection of local anesthetics, the spread of local anesthetics should be observed with ultrasound.2 We used rocuronium for tracheal intubation and sugammadex to reverse muscle relaxants for nerve stimulation with nerve blocks. Some options to control neuromuscu function are proposed for intraoperative neural monitoring,6 which can be used for nerve blocks but with some drawbacks. The use of succinylcholine enables early recovery from muscle paralysis, but it has some undesirable side effects.5,6 Lower dose of rocuronium may provide an acceptable intubating condition and early recovery from muscle relaxants, but the intubating condition may be worse.6 A combination of rocuronium and sugammadex may be optimal.6 In 1 patient, response to nerve stimulation was weak despite the adequate recovery of neuromuscular function (T1 > 50%). The reason for failure was unclear. Stimulation guidance may be ineffective in some patients.2 Needle position during the ultrasound was adequate, and ultrasound guidance was useful in this patient. Coughing and movement during positioning were adverse events caused by inadequate neuromuscular block. To minimize these events, adequate depth of anesthesia must be maintained. Titration of a lower dose of sugammadex6 can be an option to prevent these. Our protocol aimed to reverse neuromuscular block to some extent for nerve stimulation during nerve block, not to reverse it completely for extubation. The timing and dose of sugammadex we used are not enough for the complete reversal of profound neuromuscular block. Residual neuromuscular block and recurarization after surgery may be potential complications with this protocol, though they were not noted in our patients. Careful neuromuscular and clinical monitoring is essential to detect them after surgery.
We conclude that our protocol can be an option in some cases requiring peripheral nerve block under general anesthesia with tracheal intubation and nerve stimulation guidance combined with ultrasound. It may be indicated in uncooperative patients, such as children and patients with dementia or intellectual disability, and patients who strongly desire to receive the block under general anesthesia. This study involved a small number of patients, and further comparative studies are needed to evaluate the usefulness of our protocol.
Acknowledgments
We thank Nihon Kohden Corporation for the technical support of neuromuscular monitoring using the TOF-Watch® SX. We would like to thank Enago (www.enago.jp) for the English language review.
Conflict of Interest
None declared. Support was provided solely from institutional and/or departmental sources.
Registration
UMIN Clinical Trials (UMIN000027916).
References
| 1 |
Neal JM, Bernards CM, Hadzic A, et al.
ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine.
Reg Anesth Pain Med. 2008;33(5):404-415.
|
| 2 | |
| 3 |
Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J.
Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision.
Acta Anaesthesiol Scand. 2007;51(7):789-808.
|
| 4 |
Lu IC, Wu CW, Chang PY, et al.
Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve.
Laryngoscope. 2016;126(4):1014-
1019.
|
| 5 |
Sørensen MK, Bretlau C, Gätke MR, Sørensen AM, Rasmussen LS.
Rapid sequence induction and intubation with rocuronium–sugammadex compared with succinylcholine: a randomized trial.
Br J Anaesth. 2012;108(4):682-689.
|
| 6 |
Lu IC, Wu SH, Wu CW.
Neuromuscular blockade management for intraoperative neural monitoring.
Kaohsiung J Med Sci. 2020;36(4):230-235.
|