Abstract
Background: Dexmedetomidine, an α2‑adrenoreceptor agonist has been successfully used for attenuating stress response to laryngoscopy. The present study was designed to evaluate the effects of intramuscular dexmedetomidine 30 minutes before extubation on hemodynamic response in patients undergoing laminectomy for prolapsed intervertebral disc (PIVD) under endotracheal intubation and general anesthesia.
Methods: Present double‑blinded randomized placebo‑controlled study, included 100 patients from either sex with American Society of Anesthesiologists grades I and II and age from 18 to 60 years undergoing laminectomy for PIVD under general anesthesia. Patients were randomly divided into two groups of 50 each based on computer generated random numbers. The study group received dexmedetomidine (2.0 μg/kg, i.m.) in 2.5 mL saline, and the control group received normal saline (placebo, i.m.) 2.5 mL. Drugs had been administered 30 minutes before anticipated time of extubation intramuscularly at the deltoid region. Comparison of continuous variables between two groups was done by using student’s unpaired t-test. Categorical data were analyzed by using chi‑square test and Fischer Exact test as applicable.
Results: Heart rate was found to be signifi cantly lower in the study group, 15 minutes before extubation (P = 0.003), during and after extubation (P < 0.0001). The systolic and diastolic blood pressure was significantly lower in the study group during and after extubation (P < 0.05). Cough was significantly lower in the study group during extubation. No signifi cant difference was observed in other complications (respiratory stridor, incidence of laryngospasm or bronchospasm and reintubation) between the two groups. Pain score at 5 minutes, 2 hours, and 4 hours post‑extubation was signifi cantly lower in the study group (P < 0.01). P‑value < 0.05 was considered statistically significant.
Conclusion: Dexmedetomidine provides haemodynamic stability during extubation and post‑extubation. It also provides post‑operative calmness and reduces analgesic requirement and post‑extubation complications.
Keywords
dexmedetomidine, hemodynamics, intramuscular
Introduction
Endotracheal intubation is performed to protect the airway and to provide intermittent positive pressure ventilation (IPPV) in anesthetized and paralyzed patients undergoing various surgical procedures. After completion of the surgical procedures, anesthesia is terminated, residual effect of muscle relaxant is neutralized, and tracheal extubation is performed. Endotracheal extubation may be associated with sympathetic stimulation and lead to tachycardia and hypertension.1 These responses may produce myocardial ischemia, cardiac decompensation, pulmonary oedema, and cerebral haemorrhage in susceptible patients.
Dexmedetomidine, an α2‑adrenoreceptor agonist with a distribution half-life of approximately 6 minutes has been successfully used for attenuating the stress response to laryngoscopy.2,3 Currently, dexmedetomidine is used for sedation in mechanically ventilated patients in intensive care unit and for sedation of non-intubated patients before or during surgical procedures under regional anesthesia.4
A number of studies reported about the successful use of different doses of dexmedetomidine administration in the prevention of stress-induced haemodynamic changes in anesthesia practice.5-9 Moreover, different routes of α-2 agonists administration like intrathecal, epidural, nerve blocks, and intravenous have been reported to be successful in maintaining the haemodynamics.10-13
There is a paucity of studies regarding the effectiveness of intramuscular administration of dexmedetomidine on post-extubation haemodynamic response. The present study was designed to evaluate the effects of intramuscular dexmedetomidine administered 30 minutes before extubation on haemodynamic response and postoperative sedation and analgesia requirement in patients undergoing laminectomy for prolapsed intervertebral disc (PIVD) under general anesthesia and endotracheal intubation.
Methods
The present study is a double blinded randomized placebo controlled study. A total of 100 patients from either sex with American Society of Anesthesiologists (ASA) grades I and II and age from 18 to 60 years undergoing elective laminectomy for PIVD under general anesthesia and endotracheal intubation were included in the study. The study was conducted in the Department of Anesthesiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, from September 2014 to September 2016 after obtaining approval from Institutional Ethical Committee (IEB code: 2014-76-MD-76). The patients were recruited after taking signed written informed consent. The manuscript adheres to the applicable Consolidated Standards of Reporting Trials guidelines.
The patients with ASA grades III and IV, anticipated difficult airway (e.g., Mallampati class III and IV and with compromised cardiac, respiratory, renal, or hepatic functions) were excluded. Moreover, patients receiving regional anesthesia or surgery lasting more than 4 hours, smokers, blood loss more than 20% of blood volume, and requiring postoperative ventilation were also excluded from the study.
The patients were randomly divided into two groups of 50 each based on the computer generated random numbers. To ensure the double-blind nature of the study, an anesthesiology resident, who was not participated in the study, prepared the study drugs into ready-to-use form. All drugs had been administered 30 minutes before the anticipated time of extubation intramuscularly at the deltoid region.
(1) Study group (n = 50): Patients received dexmedetomidine (2.0 μg/kg, i.m.) in 2.5 mL saline.
(2) Control group (n = 50): Patients received normal saline (placebo, i.m.) 2.5 mL.
Premedication
All patients were given oral lorazepam (2 mg) and ranitidine (150 mg) in the night before surgery. In the morning of surgery, they were given oral ranitidine (150 mg) at 6:00 am. In the operation room after institution of intravenous line, midazolam (0.05 mg/kg) was administered intravenously about 5 minutes before induction of anesthesia.
Induction
In the operation room, all standard monitors like electrocardiogram, pulse oximetry (SpO2), and non-invasive blood pressure (BP) were attached to the patients. Patients were pre-oxygenated for 3 minutes on a face mask, and anesthesia was induced with a sleep dose of propofol (1.5–2.0 mg/kg) and fentanyl (2.0 μg/kg). A non-depolarizing muscle relaxant, vecuronium bromide (0.12 mg/kg), was administered intravenously to facilitate endotracheal intubation and surgical muscle relaxation. IPPV of lungs was instituted using air-oxygen mixture 50:50. All patients, irrespective of the groups, received Ringer’s lactate solution (20 mL/kg) in the first hour of anesthesia and 10 mL/kg/hr in subsequent hours. Mechanical ventilation of lungs was continued with a mixture of 50% air in oxygen and isoflurane (1%). Tidal volume and respiratory rate were adjusted to maintain normocarbia (end tidal CO2 35–40 mmHg).
During the maintenance of anesthesia, intermittent doses of fentanyl and vecuronium bromide were administered. The timing of fentanyl usage was recorded. It was avoided during the last 1 hour of the anticipated time of extubation. Urinary catheter and nasopharyngeal temperature probes were placed after endotracheal intubation to monitor urinary output and temperature, respectively. Surgical blood loss was replaced with intravenous crystalloid and colloids. The patients who incurred intra-operative blood loss of more than 20% were excluded from the study. All patients were given intravenous paracetamol (1,000 mg) within 30 minutes of commencement of surgical incision and repeated every 6 hourly postoperatively for the first 24 hours.
The anesthesia resident who was not involved in anesthesia management prepared the study drugs in 2.5 mL solution and gave intramuscularly at the deltoid region approximately 30 minutes before the endotracheal extubation. At the end of the surgery, anesthesia was terminated, and residual effect of muscle relaxant was reversed with a mixture of neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg, intravenously. On reversal of the residual effect of muscle relaxant and satisfactory respiratory parameters, endotracheal extubation was performed.
Measurements
An anesthesia resident who was not aware of the assigned patient group/drug recorded all the measurements. The haemodynamic status (heart rate, systolic blood pressure, diastolic blood pressure, and mean arterial pressure) measured 30 minutes before extubation (just before administration of dexmedetomidine) were taken as baseline haemodynamic parameters. On extubation haemodynamic parameters (heart rate, arterial blood pressure, respiratory rate, SpO2, and temperature), post extubation cough, respiratory stridor, incidence of laryngospasm or bronchospasm, and reintubation, if any of them were recorded. Standard monitoring was done in the postanesthesia care unit for 2 hours. Heart rate, systolic BP, diastolic BP, mean arterial BP, SpO2, and temperature were measured. Oxygen was supplemented if SpO2 dropped below 95%.
Modified Aldrete score was measured at every 10 minutes and time taken to achieve a score of 10 was noted in the postoperative care area. Sedation was measured by “Observer’s assessment of sedation score” on 1–5 scale. Postoperative pain was measured by numerical rating scale (NRS) on 0–10 scale immediate postoperatively, 1 hour, 2 hours, and subsequently every 4 hourly for the next 24 hours of the surgery.
Rescue analgesia in the form of tramadol was given intravenously if NRS score was equal to or more than 5. The number of rescue analgesia given was noted in each patient’s chart. The acceptable timing of the first rescue dose was 5 minutes post extubation. The interval between subsequent rescue doses was 30 minutes for the initial 2 hours, then 120 minutes for the next 2 hours, and then 240 minutes for the next 20 hours. The maximum dose decided was 400 mg tramadol in 24 hours.
Statistical Analysis
The data were entered into Statistical Package for Social Sciences (SPSS version 17.0; SPSS Inc., Chicago, IL, USA). Comparison of continuous variables between the two groups were analysed by using student’s unpaired t-test. The categorical data were analysed by using chi-square test and Fischer Exact test as applicable. P-value < 0.05 was considered as statistically significant. A sample size of 50 patients per group was needed to detect an intergroup difference of at least 10% in blood pressure and heart rate with a power of 0.80 and 5.00% type I error.
Results
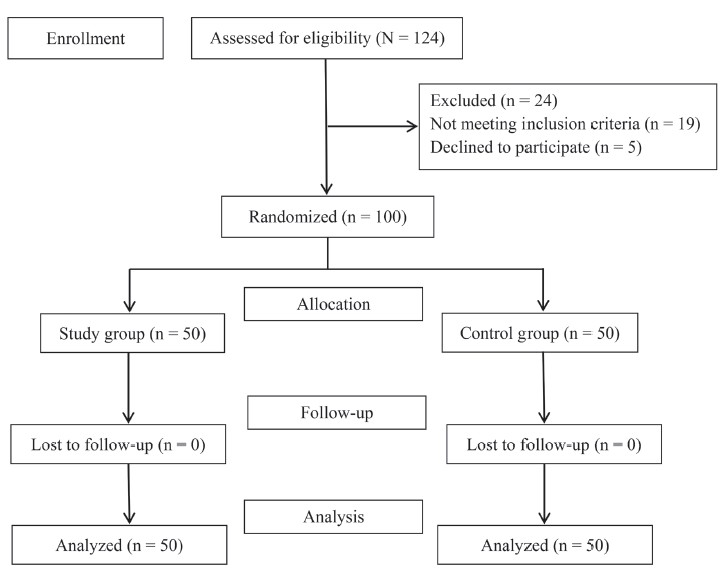
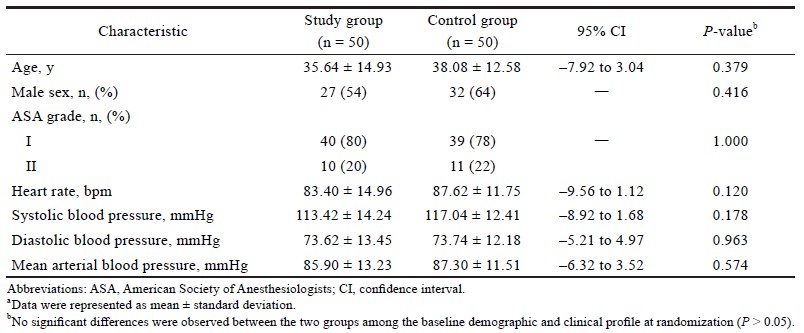
The details of patients screened for enrolment, recruited, and analysed are shown in Figure 1. The mean ± standard deviation of age of the patients was found to be comparable between the study group (35.64 ± 14.93 years) and the control group (38.08 ± 12.58 years) (95% confidence interval, –7.92 to 3.04; P = 0.379). The ratio of male patients: female patients were comparable between the study group (27:23) and the control group (32:18); P = 0.416. No significant difference was observed between the ratio of ASA I: ASA II in the study group (40:10) and the control group (39:11); P = 1.000.
Download full-size image
Total of 124 patients were assessed for eligibility. Twenty four were excluded from the study. Total of 100 patients were enrolled for the study. Fifty patients were randomized into the study group and 50 into the control group. None were lost to follow up.
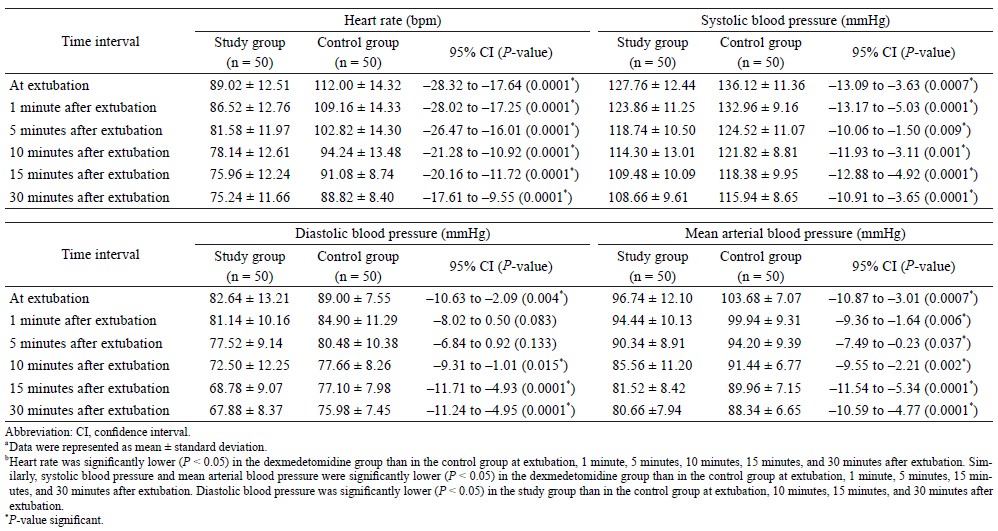
The heart rate was found to be significantly lower in the study group as compared to the control group, during and after extubation (P < 0.0001).The heart rate was found to be comparable between the study group and the control group only at 30 minutes before extubation (e.g., at baseline) (Table 1).
Download full-size image
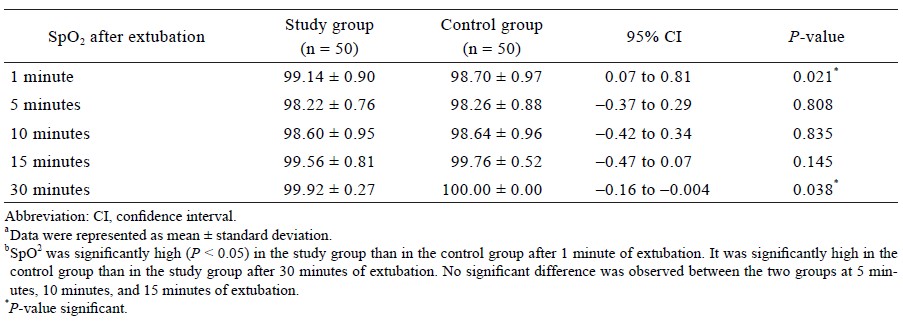
The systolic blood pressure was significantly lower in the study group as compared to the control group during and after extubation (P < 0.01) (Table 2). The diastolic blood pressure was significantly lower in the study group as compared to the control group during extubation and 10 minutes after extubation (P < 0.05) (Table 2). The mean arterial blood pressure was significantly lower in the study group as compared to the control group during and after extubation (P < 0.05) (Table 2). SpO2 was significantly higher in the study group as compared to the control group at 1 minute after the extubation (P = 0.021) (Table 3).
Download full-size image
Download full-size image
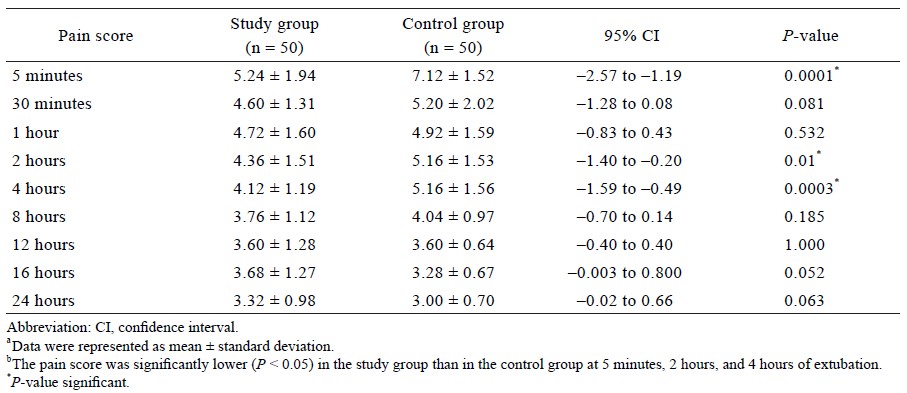
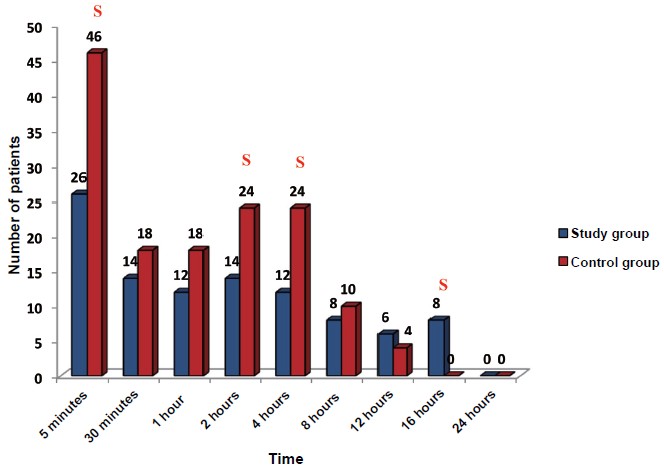
Cough was significantly lower in the study group (20%) as compared to the control group (66%) during extubation. No significant difference was observed in other complications (respiratory stridor, incidence of laryngospasm or bronchospasm, and reintubation) between the study group and the control group. The pain score on NRS at 5 minutes, 2 hours, and 4 hours post extubation was significantly lower in the study group than in the control group (P < 0.01) (Table 4). The rescue dose (tramadol 50 mg) required 5 minutes, 2 hours, and 4 hours after extubation was significantly higher in the control group than in the study group (P < 0.0001, P = 0.021, respectively). However, the rescue dose after 16 hours was required by 16% of patients in the study group as compared to 0% of patients in the control group (P = 0.006) (Figure 2).
Download full-size image
Download full-size image
The number of patients requiring rescue dose at 5 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours were high in the control group than in the study group.
Abbreviation: S, signifi cant.
No difference was observed in pain score at discharge between the study group and the control group (P > 0.05). The time taken to achieve an Aldrete score of 10 was significantly higher in the study group (13.20 ± 4.48 minutes) than in the control group (10.72 ± 3.12 minutes) (P = 0.002). The sedation score was found to be comparable at 5 minutes and at 30 minutes after extubation between the study group (3.72 ± 0.45 and 4.88 ± 0.33, respectively) and the control group (3.76 ± 0.72 and 4.80 ± 0.40, respectively); P > 0.05.
Discussion
The different routes of α-2 agonists administration like intrathecal, epidural, nerve blocks, and intravenous have been reported to be successful in maintaining the haemodynamics.10-13 Few studies have reported the intramuscular administration of dexmedetomidine to premedicate the surgical patients.14,15 The study by Kallio et al.16 reported that the intramuscular route of administration of α-adrenergic drugs results in smoother haemodynamic response than intravenous administration. Therefore, in the present study, the intramuscular route was selected for the administration of dexmedetomidine.
The results of the present study showed that intramuscular dexmedetomidine was given 30 minutes before extubation provides haemodynamic stability during and after extubation. The haemodynamic variables (heart rate, systolic blood pressure, diastolic blood pressure, and mean blood pressure) showed a significant decrease in the dexmedetomidine group compared to the control group during and after the extubation.
The study by Kallio et al.16 showed dose-dependent decreases in systolic and diastolic blood pressure and also in heart rate when administered to 5 healthy males in single doses of 12.5, 25.0, 50.0, and 75.0 μg as part of a placebo-controlled study. Aho et al.17 investigated the effects of three different doses of dexmedetomidine (0.6, 1.2, and 2.4 μg/kg), or oxycodone (0.13 mg/kg), or saline solution, injected intramuscularly on haemodynamic in laparoscopy gynaecological diagnostic procedures and found that the arterial blood pressure and heart rate were lower in dexmedetomidine group compared to saline group. Another study done by Basar et al.18 showed that 0.5 μg/kg dexmedetomidine on induction significantly decreases the heart rate compared to saline solution.
Kaya et al.19 reported that the values in heart rate and mean arterial pressure increased after intubation and extubation compared to baseline values in the control group while in dexmedetomidine group heart rate, mean arterial pressure was similar to the baseline values. Sarpkaya et al.20 investigated the effects of dexmedetomidine (loading dose of dexmedetomidine [1.0 μg/kg] and maintenance of [0.5 μg/kg/h]) on hemodynamic changes and found that systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate values were significantly lower in dexmedetomidine group compared to control.
In the present study, it was found that the pain score on NRS after the extubation was significantly lower in the dexmedetomidine group than in the control group. Consequently, the analgesic requirement was also significantly lower in the dexmedetomidine group than in the control group. Similar to present findings, Arain et al.21 also reported that patients who received intraoperative dexmedetomidine infusion (0.4 μg/kg/h) after initial i.v. loading dose (1.0 μg/kg over 10 minutes), required 66% less morphine. Another study by Venn and Ground22 also showed that the requirements for morphine were reduced by more than 50% in patients receiving dexmedetomidine when compared with placebo after extubation (0.003 ± 0.004 vs. 0.008 ± 0.006 mg/kg/h; P = 0.040). The similar finding was reported by Abdel-Meguid23 that the mean total use of narcotics in group II (saline) was 23.5 ± 20.7 mg compared to 11.4 ± 6.3 mg in group I (dexmedetomidine). Panchgar et al.24 reported that analgesic requirement during postoperative 24 hours were much less in the dexmedetomidine group when compared to normal saline group.
In the present study, the post-extubation complication (e.g., cough) was significantly lower in the study group (20%) than in the control group (66%). However, no significant difference was observed in other complications (respiratory stridor, incidence of laryngospasm or bronchospasm, and reintubation) between the two groups. The study by Bindu et al.25 also reported similar findings of moderate coughing in 16% of patients who received an intravenous infusion of dexmedetomidine 0.75 mcg/kg compared to 84% of patients in the placebo group.
In the present study, the sedation score was found to be comparable at 5 minutes and at 30 minutes after extubation between the study group (3.72 ± 0.45 and 4.88 ± 0.33, respectively) and the control group (3.76 ± 0.72 and 4.80 ± 0.40, respectively) (P > 0.05). However, the study by Bindu et al.25 reported a significant difference in the level of postoperative sedation between the dexmedetomidine and placebo groups (P = 0.017). The study by Patel et al.26 showed that postoperatively, the test group showed signifi cant sedation at 2 horus as compared to the control group (P = 0.00). The difference in the results can be due to the route of administration of the drug. The route of administration of dexmedetomidine in the studies by Bindu et al.25 and Patel et al.26 was intravenous. However, in our study the route was intramuscular. This could have led to the difference in the results.
Dexmedetomidine provides haemodynamic stability during extubation and post extubation. It also provides post-operative calmness, reduces analgesic requirement, and post extubation complications. However, until the haemodynamic stability of lowdose i.m. dexmedetomidine is demonstrated in a large-scale multicentre trial, it is prudent to monitor carefully for potential ad-verse effects in all treated patients.
References
| 1 |
Swamy SN, Madhusudhana R.
Attenuation of hemodynamic
responses to endotracheal extubation with diff erent
doses of diltiazem with lignocaine: a placebo-controlled
study.
Anesth Essays Res. 2018:12(2):428-433.
|
| 2 | |
| 3 |
Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS,
Ravullapalli H, Gandham R.
The effects of dexmedetomidine
on attenuation of stress response to endotracheal
intubation in patients undergoing elective off-pump
coronary artery bypass grafting.
Ann Card Anaesth.
2012;15(1):39-43.
|
| 4 | |
| 5 |
Feld JM, Hoff man WE, Stechert MM, Hoff man IW, Ananda
RC.
Fentanyl or dexmedetomidine combined with desfl
urane for bariatric surgery.
J Clin Anesth. 2006;18(1):24-
28.
|
| 6 |
Ramsay MA, Saha D, Hebeler RF.
Tracheal resection in
the morbidly obese patient: the role of dexmedetomidine.
J Clin Anesth. 2006;18(6):452-454.
|
| 7 |
Tufanogullari B, White PF, Peixoto MP, et al.
Dexmedetomidine
infusion during laparoscopic bariatric
surgery: the effect on recovery outcome variables.
Anesth Analg. 2008;106(6):1741-1748.
|
| 8 |
Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K.
The
effect of intravenously administered dexmedetomidine on
perioperative hemodynamics and isofl urane requirements in patients undergoing abdominal hysterectomy.
Anesthesiology.
1991;74(6):997–1002.
|
| 9 |
Keniya VM, Ladi S, Naphade R.
Dexmedetomidine attenuates
sympathoadrenal response to tracheal intubation
and reduces perioperative anaesthetic requirement.
Indian
J Anaesth. 2011;55(4):352-357.
|
| 10 |
Villela NR, Nascimento Júnior Pd.
Dexmedetomidine in
anesthesiology.
Rev Bras Anestesiol. 2003;53(1):97-113.
|
| 11 |
Rai A, Bhutia MP.
Dexmedetomidine as an additive
to spinal anaesthesia in orthopaedic patients undergoing
lower limb surgeries: a randomized clinical trial
comparing two different doses of dexmedetomidine.
J
Clin Diagn Res. 2017;11(4):UC09-UC12.
|
| 12 |
Schnabel A, Reichl SU, Weibel S, et al.
Efficacy and
safety of dexmedetomidine in peripheral nerve blocks:
a meta-analysis and trial sequential analysis.
Eur
J Anaesthesiol. 2018;35(10):745-758.
|
| 13 |
Zeng X, Jiang J, Yang L, Ding W.
Epidural dexmedetomidine
reduces the requirement of propofol during total
intravenous anaesthesia and improves analgesia after
surgery in patients undergoing open thoracic surgery.
Sci
Rep. 2017;7(1):3992.
|
| 14 |
Karaaslan D, Peker TT, Alaca A, et al.
Comparison of
buccal and intramuscular dexmedetomidine premedication
for arthroscopic knee surgery.
J Clin Anesth.
2006;18(8):589-593.
|
| 15 |
Erkola O, Korttila K, Aho M, Haasio J, Aantaa R, Kallio A.
Comparison of intramuscular dexmedetomidine
and midazolam premedication for elective abdominal
hysterectomy.
Anesth Analg. 1994;79(4):646-653.
|
| 16 |
Kallio A, Scheinin M, Koulu M, et al.
Effects of dexmedetomidine,
a selective alpha 2-adrenoceptor agonist, on hemodynamic
control mechanisms.
Clin Pharmacol Ther.
1989;46:33-42.
|
| 17 |
Aho M, Scheinin M, lehtinen AM, Erkola O, Vuorinen
J, Korttila K.
Intramuscularly administered dexmedetomidine
attenuates hemodynamic and stress hormone
responses to gynecologic laparoscopy
Anesth Analg.
1992;75(6):932-939.
|
| 18 |
Basar H, Akpinar S, Doganci N, et al.
The effects of preanesthetic, single-dose dexmedetomidine on induction,
hemodynamic, and cardiovascular parameters.
J Clin Anesth. 2008;20(6):431-436.
|
| 19 |
Kaya C, Kelsaka E, Sarhasan B, Yazcoğlu AY.
Does dexmedetomidine
premedication have an effect on stress
response?: A-603.
Eur J Anaesthesiol. 2006;23(Suppl
37):156-157.
|
| 20 |
Sarpkaya A, Karaaslan K, Kocoglu H, Bugdayci G, Colak
C.
The effects of perioperative use of dexmedetomidine
on hemodynamic parameters and surgical stress response
in chronic hypertensive patients: 4AP7-8.
Eur J
Anaesthesiol. 2010;27(47):86-87.
|
| 21 |
Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ.
The
efficacy of dexmedetomidine versus morphine for
postoperative analgesia after major inpatient surgery.
Anesth Analg. 2004;98(1):153-158.
|
| 22 |
Venn RM, Ground RM.
Comparison between dexmedetomidine
and propofol for sedation in the intensive care
unit: patient and clinician perceptions.
Br J Anaesth.
2001;87(5):684-690.
|
| 23 |
Abdel-Meguid ME.
Dexmedetomidine as anesthetic
adjunct for fast tracking and pain control in off-pump
coronary artery bypass.
Saudi J Anaesth. 2013;7(1):6-8.
|
| 24 |
Panchgar V, Shetti AN, Sunitha HB, Dhulkhed VK,
Nadkarni AV.
The effectiveness of intravenous dexmedetomidine
on perioperative hemodynamics, analgesic
requirement, and side effects profile in patients undergoing
laparoscopic surgery under general anesthesia.
Anesth Essays Res. 2017;11(1):72-77.
|
| 25 |
Bindu B, Pasupuleti S, Gowd UP, Gorre V, Murthy RR,
Laxmi MB.
A double blind, randomized, controlled trial
to study the effect of dexmedetomidine on hemodynamic
and recovery responses during tracheal extubation.
J Anaesthesiol Clin Pharmacol. 2013;29(2):162-167.
|
| 26 |
Patel CR, Engineer SR, Shah BJ, Madhu S.
Effect of
intravenous infusion of dexmedetomidine on perioperative
haemodynamic changes and postoperative recovery:
a study with entropy analysis.
Indian J Anaesth.
2012;56(6):542-546.
|