Abstract
Background
Patients with alcohol drinking habits have less nausea and vomiting during chemotherapy because of cytochrome P450 enzyme induction. However, few studies have examined the effect of alcohol consumption on postoperative nausea and vomiting (PONV). We conducted a study to clarify the relationship between alcohol drinking habits and PONV.
Methods
Data of patients undergoing hepatectomy under general anesthesia between 2016 and 2020 were retrospectively collected. Since alcohol drinking habits vary by gender, age, and comorbidities, propensity score matching was performed to adjust patient background before multivariate logistic regression analysis.
Results
Seventy-eight patients in the alcohol consumption and non-consumption groups were matched by propensity matching. Univariate analysis showed that alcohol consumption (
Conclusion
Patients with no alcohol drinking habits may be at higher risk of PONV.
Keywords
alcohol consumption, hepatectomy, PONV
Introduction
Postoperative nausea and vomiting (PONV) is a major complication of general anesthesia, along with pain, and is not only uncomfortable for the patient but also a disincentive to early recovery.1,2 The frequency of PONV in general population is around 30%, but in high-risk patients, it is as high as about 70%.3,4 Therefore, numerous studies have focused on the prevention and risk stratification of PONV in past few decades. The use of inhalational anesthetic drugs, nitrous oxide, and opioids have been well documented as risk factors of PONV, and these drugs should be used minimally in high-risk patients, such as women, nonsmokers, patients with history of motion sickness, and young-aged patients.5-8 However, despite these well-known strategies, many patients still suffer from PONV. Some patients find the nausea more stressful than the pain and are willing to pay additional costs to combat PONV.9 Therefore, it is important to implement PONV strategies known to date and to seek to identify other new risk factors.
In recent years, so-called pre-habilitation programs that evaluate and justify risk factors before surgery in order to enhance early recovery have been attracting attention.10 Among them, smoking cessation and interruption of alcohol consumption are recommended in many guidelines before surgery as they improve the outcome after surgery.11 In particular, patients undergoing hepatectomy require strict abstinence from alcohol during the perioperative period because continued alcohol consumption further damages the liver and deprives the patient of treatment options such as surgery and transplantation.
On the other hand, it has long been known that cisplatin-induced nausea and vomiting is less common in patients with chronic alcohol consumption.12,13 This may be attributed to the fact that alcohol activates cytochrome P450 (CYPs) and accelerates the metabolism of cisplatin. Same hypothesis can be applied to anesthetic drugs, such as inhalational drugs and opioids.
However, to date, there has been no clear study on the incidence of PONV and alcohol consumption, except for one report in 2010 and the evidence is lacking.14 If the relationship between alcohol consumption habits and PONV is clarified, it will be useful to identify high-risk patients of PONV in advance and to consider anesthesia methods to prevent PONV.
In this study, we aimed to clarify the relationship between alcohol consumption and PONV in patients who underwent partial hepatectomy, in which a relatively high incidence of PONV and a high level of compliance with alcohol abstinence after diagnosis of the disease are observed.
Methods
This is a single-center, retrospective, and observational study. The study was approved by the medical ethics committee of Nara Medical University Hospital (approval number, 2915; date: February 21, 2021). The requirement for written informed consent was waived in view of the retrospective nature of the research. At the initiation of the study, eligible patients were given the option to opt-out of having their data removed from the study. In brief, the opt-out document approved by the ethics committee was submitted on the web page. The period and procedures to be included in the study were described in detail so that patients could identify whether they were eligible for the study, and the contact information for withdrawing from the study was also clearly stated. Patients who underwent partial hepatectomy in our hospital between April 2016 and December 2020 were eligible. The events of PONV were extracted from the records of postoperative anesthesia clinic examination records. The postoperative examination was performed approximately 7 days after surgery by face-to-face consultation with an anesthesiologist. In the medical records, PONV was classified as (1) not to present at all, (2) to present only on the day of the surgery, or (3) to present on the following day or later. We defined PONV group as patients with (2) or (3). In addition, the presence or absence of PONV was confirmed by referring to the electronic medical record, and cases in which PONV was recorded in the medical record were treated as cases with PONV even if they were not classified as (2) or (3) in the postoperative anesthesia clinic.
Age, gender, height, weight, body mass index, and smoking history (never smoked, quit smoking, and still smoking) were collected as patient background. History of cerebrovascular disease, asthma, hypertension, diabetes mellitus, and ischemic heart disease were collected as past medical history. We also collected serum creatinine level and serum albumin level as blood test findings. Anesthesia time, anesthesia method (inhalation or total intravenous anesthesia [TIVA]), total dose of fentanyl, remifentanil, use of antiemetic drugs, and intraoperative total balance were extracted from electronic anesthesia records. In this study, alcohol consumption habits were categorized as daily drinking, abstinence within 3 months, abstinence for more than 3 months, occasional drinking, or never drinking alcohol. Patients who drank every day or abstained from alcohol for less than 3 months were categorized as the alcohol consumption group, and those who drank occasionally, abstained from alcohol for more than 3 months, or did not drink at all were categorized as the non-alcohol consumption group.
Statistical Analysis
Since alcohol consumption habits vary depending on age, gender, preoperative conditions (presence of underlying diseases such as heart failure and diabetes), and smoking history, propensity score matching was conducted to eliminate these biases. In other words, propensity scores were calculated by multiple logistic regression analysis with the presence or absence of alcohol consumption as the objective variable. The created logistic regression model was examined by Hosmer-Lemeshow test to check its validity. After the validation of the regression model, 1 : 1 matching was performed by using the nearest neighbor method, allowing for a 15% error in the standard deviation of the propensity score (caliper = 0.15). Multiple logistic regression analysis was then performed with PONV as the objective variable in the adjusted cohort.
Sample Size Calculation
We assumed that the incidence of PONV is 25% and that there are six factors that influence alcohol consumption: age, gender, preoperative complications (hypertension and diabetes), nutritional status (serum albumin level), and smoking habits. The number of cases required for logistic regression analysis is calculated to be 1 / 0.25 ×10 × 6 = 240. Since about 50 partial hepatectomies are performed annually in our institute, we collected data for the past 5 years (2016–2020). Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as number of cases (%). All statistical processing was performed by using R version 4.0.4 (R foundation for statistical computing, Vienna, Austria), and a
Results
During the study period, there were 261 eligible patients; 4 patients were excluded because of missing preoperative alcohol consumption records. There was no emergency surgery, and all patients received a preoperative anesthesiology consultation. Therefore, 257 cases were included in the final analysis.
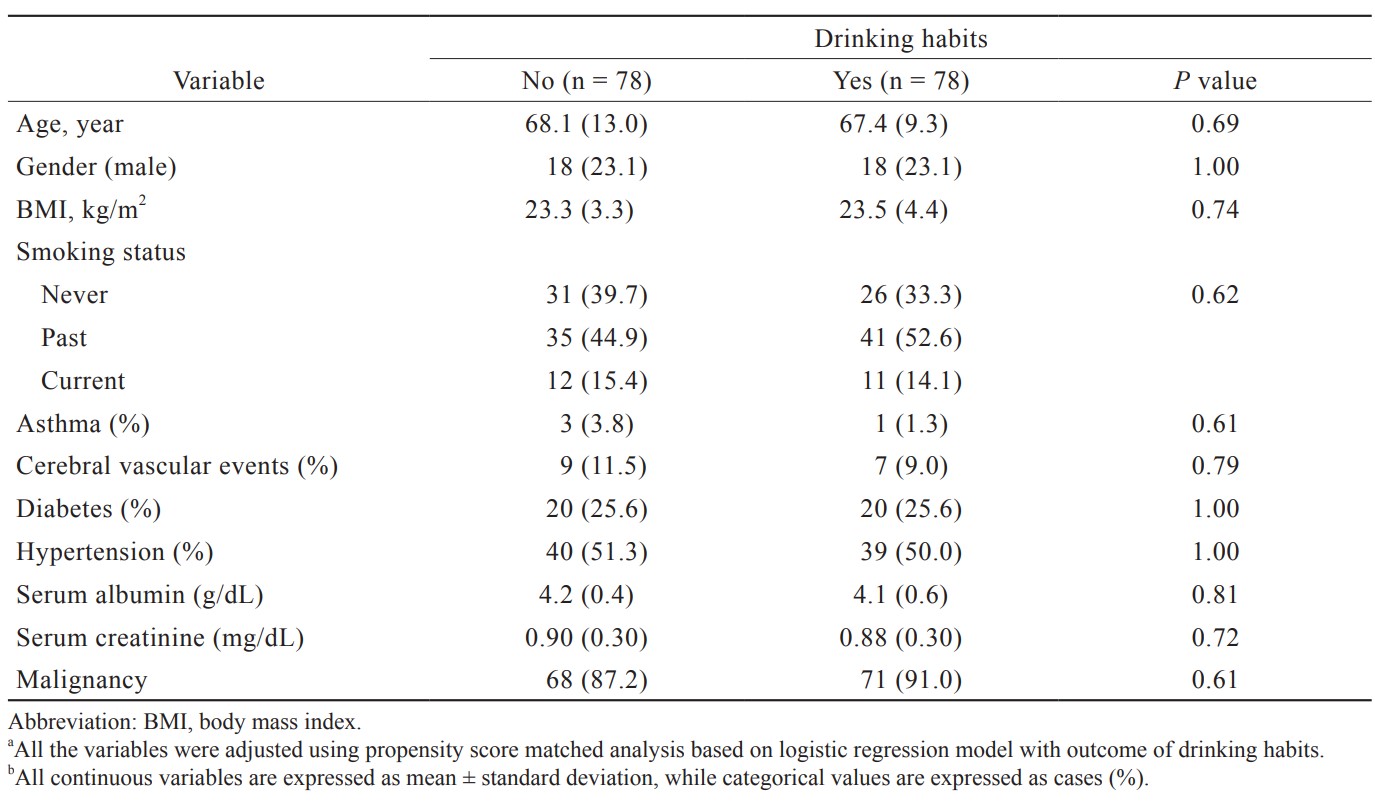
Of the 257 cases, 67 (26.1%) cases had PONV. One-hundred and thirty-three patients (51.0%) were classified as the alcohol consumption group. The results between the two groups before being adjusted for background variables are shown in Table 1. The factors that influenced alcohol consumption were gender, height, weight, and smoking history. In order to adjust the patients’ backgrounds, multiple logistic regression analysis was performed with alcohol consumption as the objective variable, and propensity score matching was performed. The validity of the developed model was confirmed by the Hosmer–Lemeshow test, with the result of
Download full-size image
Download full-size image
Download full-size image
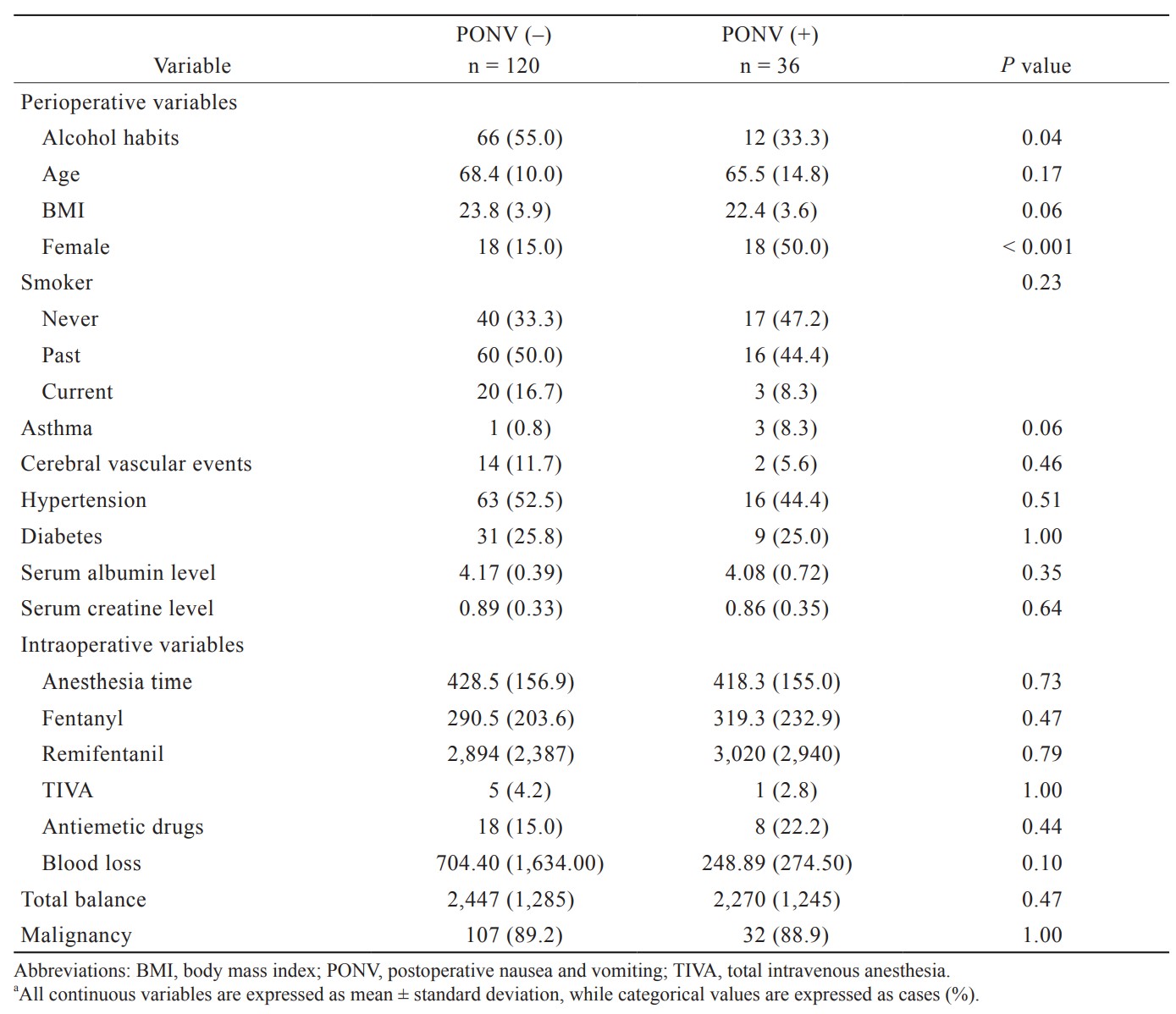
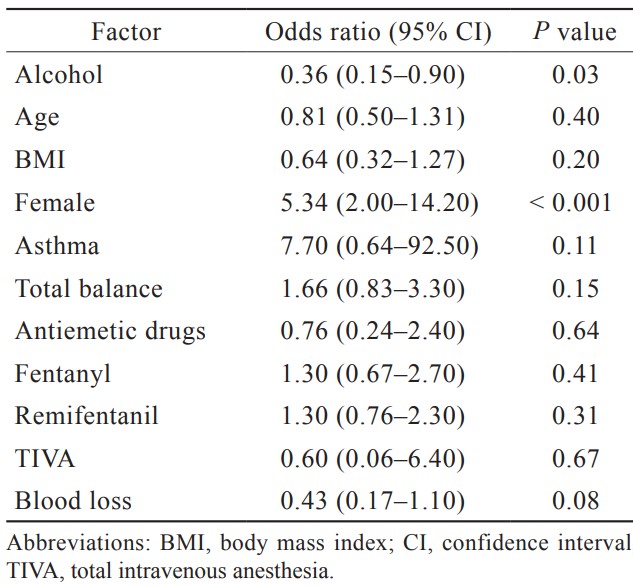
Multiple logistic regression analysis with PONV as the outcome was performed by using the matched data. Perioperative variables with
Download full-size image
Discussion
The present study is the first to evaluate the effect of alcohol consumption on PONV. Although a randomized controlled trial (RCT) is usually preferred to examine the effect of an intervention on an outcome, habits such as alcohol consumption or smoking is not randomizable. Furthermore, RCTs have a few disadvantages. They are difficult to be generalized because they include only patients who meet specific inclusion criteria, and crossover after randomization usually makes the results difficult to interpret. In such situations, propensity score matching is useful. In the current study, propensity score matched analysis revealed that preoperative alcohol consumption was significantly associated with decreasing the incidence of PONV.
It has been pointed out that small amounts of alcohol consumption decreases the incidence of cardiovascular events, while large amounts of alcohol consumption increases the incidence of the events and the relationship is so-called J Curve.15 For this reason, anesthesiologists tend not to be as concerned about alcohol consumption as we are about bad habits such as smoking. However, hazardous drinking (36 g ethanol/day) defined by World Health Organization is found in 7%–49% of patients undergoing scheduled surgery and 14%–38% of patients undergoing emergency surgery.16 Perioperative negative effects of alcohol have been reported to include liver dysfunction, central nervous system disorders such as delirium and seizures, cardiovascular disorders such as arrhythmias and decreased cardiac function, and impairment of cellular immune system. Therefore, it is important to discontinue alcohol use in the perioperative period. However, the results of this study showed that the incidence of PONV was higher in patients who had interrupted alcohol use 3 months before the surgery and in those who were not originally in the habit of drinking. Therefore, it is important to recognize these groups of patients as high-risk patients of PONV and to promote countermeasures.
The metabolism of drugs, including anesthetics, is mediated by CYPs enzymes. For example, the metabolism of fentanyl and rocuronium is metabolized by CYP3A4.17 Although the metabolism rates of inhaled anesthetics
Apfel et al.5 cited women as a factor in their famous PONV risk score. When this report was published in 1990s, women were less likely than men to be habitual drinkers and the consumption amount of alcohol remained low. However, recent study has pointed out that alcohol consumption by women is catching up with men in past few decades.21 We hypothesized that there might be no difference in the incidence of PONV between men and women if patients’ characteristics including alcohol consumption was adjusted. However, women were still a significant factor after propensity score matching by alcohol consumption. Therefore, impact of gender difference on PONV might be due to the differences of the genetic level (e.g., differences in the phenotype of OPRM1, the gene encoding fentanyl) rather than the socio-behavioral differences.22
In this study, there was no difference in factors that have been reported to be associated with PONV incidence in the past, such as anesthesia time, anesthesia method (inhalation or TIVA), and intraoperative balance. This may be due to the fact that there was no significant difference in anesthesia time or fluid volume for the single procedure of liver surgery. TIVA was applied in only 4.6% of the cases, and the remaining cases were maintained with inhalational anesthesia. The heterogeneity of the anesthetic technique might be responsible for the results.
Finally, the limitations of this study are discussed. In our study, we were not able to collect other well-known factors to be associated with PONV, such as history of motion sickness. The second limitation of this study is that we collected alcohol consumption data from medical records retrospectively. It has been previously pointed out that evaluation of alcohol consumption based on questionnaires may be at risk of underestimation.23 In addition, the study was conducted with Japanese patients, which we must consider racial difference. In particular, < 50% of the Japanese are poor metabolizers of acetaldehyde, and it is unclear whether similar results can be obtained in all races.24
Conclusion
In this study, preoperative abstinence from alcohol was a risk factor for PONV in patients undergoing liver resection surgery. Further prospective studies in multiple procedures are needed to support the evidence.
Author Contributions
YY and HN: data collection and data analysis; YN: study design, writing the first draft of the paper; MI: statistical analysis, making advice for writing the first draft of the paper; MK: reviewing the first draft of the paper and rewriting. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Sharing
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
| 1 |
Eberhart LH, Högel J, Seeling W, Staack AM, Geldner G, Georgieff M.
Evaluation of three risk scores to predict postoperative nausea and vomiting.
Acta Anaesthesiol Scand. 2000;44(4):480-488.
|
| 2 |
Glass PSA, White PF.
Practice guidelines for the management of postoperative nausea and vomiting: past, present, and future.
Anesth Analg. 2007;105(6):1528-1529.
|
| 3 |
Gan TJ.
Postoperative nausea and vomiting—can it be eliminated?
JAMA. 2002;287(10):1233-1236.
|
| 4 |
Phillips C, Brookes CD, Rich J, Arbon J, Turvey TA.
Postoperative nausea and vomiting following orthognathic surgery.
Int J Oral Maxillofac Surg. 2015;44(6):745-751.
|
| 5 |
Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N.
A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers.
Anesthesiology. 1999;91(3):693-700.
|
| 6 |
Cao X, White PF, Ma H.
An update on the management of postoperative nausea and vomiting.
J Anesth. 2017;31(4):617-626.
|
| 7 |
Öbrink E, Jildenstål P, Oddby E, Jakobsson JG.
Post-operative nausea and vomiting: update on predicting the probability and ways to minimize its occurrence, with focus on ambulatory surgery.
Int J Surg. 2015;15:100-106.
|
| 8 |
Leslie K, Myles PS, Chan MTV, et al.
Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide-based vs nitrous oxide-free anaesthesia.
Br J Anaesth. 2008;101(4):498-505.
|
| 9 |
Sizemore DC, Singh A, Dua A, Singh K, Grose BW.
Postoperative nausea.
|
| 10 |
Kawaguchi M, Ida M, Naito Y.
The role of perioperative surgical home on health and longevity in society: importance of the surgical prehabilitation program.
J Anesth. 2017;31(3):319-324.
|
| 11 |
Tønnesen H, Nielsen PR, Lauritzen JB, Møller AM.
Smoking and alcohol intervention before surgery: evidence for best practice.
Br J Anaesth. 2009;102(3):297-306.
|
| 12 |
Sullivan JR, Leyden MJ, Bell R.
Decreased cisplatin-induced nausea and vomiting with chronic alcohol ingestion.
N Engl J Med. 1983;309(13):796.
|
| 13 |
Sekine I, Segawa Y, Kubota K, Saeki T.
Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis.
|
| 14 |
Morino R, Ozaki M, Nagata O, Yokota M.
Incidence of and risk factors for postoperative nausea and vomiting at a Japanese cancer center: first large-scale study in Japan.
J Anesth. 2013;27(1):18-24.
|
| 15 |
Thompson PL.
J-curve revisited: cardiovascular benefits of moderate alcohol use cannot be dismissed.
Med J Aust. 2013;198(8):419-422.
|
| 16 |
Tønnesen H.
Alcohol abuse and postoperative morbidity.
Dan Med Bull. 2003;50(2):139-160.
|
| 17 | |
| 18 |
Kharasch ED.
Metabolism and toxicity of the new anesthetic agents.
Acta Anaesthesiol Belg. 1996;47(1):7-14.
|
| 19 |
Kharasch ED, Thummel KE.
Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane.
Anesthesiology. 1993;79(4):795-807.
|
| 20 |
Blomqvist O, Ericson M, Johnson DH, Engel JA, Söderpalm B.
Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment.
Eur J Pharmacol. 1996;314(3):257-267.
|
| 21 |
Erol A, Karpyak VM.
Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations.
Drug Alcohol Depend. 2015;156:1-13.
|
| 22 |
Pang GSY, Ithnin F, Wong YY, et al.
A non-synonymous single nucleotide polymorphism in an OPRM1 splice variant is associated with fentanyl-induced emesis in women undergoing minor gynaecological surgery.
PLoS One. 2012;7(11):e48416.
|
| 23 |
Etter JF, Perneger TV.
Measurement of self reported active exposure to cigarette smoke.
J Epidemiol Community Health. 2001;55(9):674-680.
|
| 24 |
Goedde HW, Agarwal DP, Fritze G, et al.
Distribution of ADH2 and ALDH2 genotypes in different populations.
Hum Genet. 1992;88(3):344-346.
|