Abstract
Background
Shivering is one of the most common complications of spinal anesthesia because of inhibition of the thermoregulatory control. Dexmedetomidine and nalbuphine are the two commonly used drugs for treatment of perioperative shivering, but owing to paucity of their comparative data, we planned this study to compare the efficacy of these two drugs for treatment of post spinal shivering.
Methods
This study was conducted on 80 American Society of Anesthesiologists physical status I or II patients aged from 18 to 60 years who developed post-spinal shivering of grade III or IV during elective surgeries. These patients were randomly allocated into two groups (40 each). In group D, dexmedetomidine 0.50 µg/kg, and in group N, nalbuphine 0.08 mg/kg was given intravenously for treatment of shivering. Data regarding response time, recurrence rate and success rate along with their adverse effects were noted, and statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
The mean response time was significantly shorter in group D as compared to Group N (1.9 ± 0.6 min and 4.7 ± 1.1 min, respectively;
Conclusions
Dexmedetomidine is a better alternative than nalbuphine for treatment of post spinal shivering with quicker response time and comparable side effects.
Keywords
dexmedetomidine, nalbuphine, regional anesthesia, shivering
Introduction
Shivering is defined as an involuntary, oscillatory activity of skeletal muscle that occurs as a physiological response to raise the metabolic heat production.1 It can usually affect up to 30%–60% of patients during central neuraxial anesthesia and 5%–65% of patients during general anesthesia.2 The main reason of post-anesthetic shivering is perioperative hypothermia which occurs because of impairment of the thermoregulatory control induced by regional anesthesia. However, shivering also occurs in normothermic patients probably because of blockage of the spinal reflex, diminished sympathetic activity, adrenal gland suppression, and pyrogen release.3
Post anesthesia shivering not only causes discomfort to the patient but also increases the oxygen consumption (200%–500%) and carbon dioxide production which ultimately lead to arterial hypoxemia, lactic acidosis, and increase in intraocular and intracranial pressure.4 Shivering also affects the monitoring of electrocardiography (ECG) and blood pressure which may increase the postoperative complications especially in high-risk patients with limited cardiorespiratory reserve.5
Post anesthesia shivering can be prevented and treated by various non pharmacological and pharmacological methods. Non pharmacological method includes covering with blankets, radiant heat application, warming the operating theatre and use of warm intravenous (IV) fluids. Although skin surface rewarming is a quick way of raising the skin temperature, it is less efficient, as it only contributes 20% to control of shivering and vasoconstriction. Pharmacological methods like ketanserin, opioids (pethidine or tramadol), propofol, granisetron, physostigmine, clonidine, dexmedetomidine, and nefopam are routinely used but with their own demerits.6 However, research on an “ideal anti-shivering drug” still continues.
Pethidine is possibly the most effective anti-shivering drug used as its anti-shivering effect is mediated by action on kappa receptor and central α2 receptors.7,8 Nalbuphine, a newer semisynthetic opioid, has the characteristic μ antagonist and κ agonist activity. Its anti-shivering property is attributed to κ agonistic action. Dexmedetomidine is highly selective α2 adrenoreceptors agonist used as sedative agent and is also known to reduce shivering threshold. As the pethidine has undesired adverse effects and difficulty in over-the-counter availability, we planned to study dexmedetomidine and nalbuphine as anti-shivering agents which are easily available especially in our country. As the comparative data regarding their use for post spinal shivering treatment are very sparse, we do this clinical trial to evaluate the efficacy of both the drugs in terms of response time, success rate, and recurrence of shivering along with their adverse effects.
Methods
This prospective, randomized, comparative trial was conducted over a period of 14 months as per the Indian Council of Medical Research rules for biomedical study in human population and in accordance with the principles of Declaration of Helsinki 2013. After taking approval from the institutional ethical committee (ECR/836/Inst/PB/2016) dated February 13, 2018 and registering the trial with the Clinical Trial Registry of India (CTRI/2018/07/014854) along with written informed consent, we enrolled 80 adult patients aged 18–60 years of either sex with American Society of Anesthesiologists (ASA) grade I or II undergoing elective surgeries (orthopedic or gynecological surgeries) who established post-spinal shivering of grade III or IV up to 2 hours of subarachnoid block. Patients with an initial abnormal body temperature (> 38°C or < 36°C), and those with a history of multiple allergies, Parkinson’s disease, hypertension, coronary artery disease, arrhythmia or neuromuscular pathology, or having contraindications to spinal anesthesia, on treatment with sedative hypnotic agents or vasodilators, and procedure requiring blood transfusion were excluded from study.
A systematic preoperative assessment of each patient was done by an anesthesiologist. All the patients were premedicated with alprazolam 0.25 mg and ranitidine 150 mg orally at night before surgery and were instructed for fasting for 6 hours as per standard guidelines before surgery. After arrival in the operative room, standard ASA monitors including ECG, pulse oximeter (SpO2), noninvasive blood pressure and axillary temperature were attached and baseline parameters were recorded. IV line with 18 G cannula secured and ringer lactate infusion at 10 mL/kg was started. Under all aseptic condition, subarachnoid block was performed at L3–L4 or L4–L5 level with 25 G Quincke’s spinal needle using 3.0–3.5 mL of 0.5% heavy bupivacaine. Patient was made supine to achieve a desirable level at T8–10 dermatomes, according to surgical procedure. All patients were protected with two layers of surgical drapes. Level of anesthesia was assessed after subarachnoid block and thereafter at every 5 minute intervals till it got fixed. Operation theatre was maintained at an ambient temperature of around 24–26°C and no other warming device was used. IV fluids and local anesthetics were given at room temperature. After induction of subarachnoid block, all patients were monitored for the occurrence of shivering according to the following grades9: (1) Grade 0—No shivering; (2) Grade I—Piloerection, peripheral vasoconstriction and cyanosis without any visible muscle activity; (3) Grade II—Visible muscular activity in only one muscle group; (4) Grade III—Visible muscular activity involving more than one muscle group but not generalized; (5) Grade IV—Gross muscle activity affecting the whole body. Patients who established post-spinal shivering grades III or IV were randomly divided into following two groups using computer generated randomization programs and allocation concealed by opaque sealed envelope containing code. For treatment of shivering, dexmedetomidine 0.5 μg/kg diluted in 10 mL normal saline (NS) over 2 minutes in group D and nalbuphine 0.08 mg/kg diluted in 10 mL NS over 2 minutes in group N was given IV. Drugs were prepared in identical syringes by an anesthesiologist according to code inside envelop who was not participating in the study.
The independent attending observer, who was also blind regarding drug administered, recorded time of shivering onset (time in minutes at which shivering started after placement of spinal anesthesia), gradation of the shivering, and time taken for complete disappearance of shivering (response time). Complete disappearance of shivering was defined when post treatment, the shivering score declined to 0 and failed when no change in scores was observed. He also noted success rate (the shivering ceased within 15 minutes after the treatment) and recurrence of shivering up to 120 minutes after spinal anesthesia. Patients of whom study drugs failed or recurrence of shivering occurred, were managed with tramadol 1 mg/kg IV. Adverse effects such as bradycardia [(< 50/min) treated with atropine (0.5 mg IV)], hypotension [(< 20% of baseline) treated with mephentermine (3.0 mg IV)], nausea, vomiting, and sedation were noted. The degree of sedation was noted on a four-point Filos et. al.10 scale. Nausea and vomiting were managed with metoclopramide 10 mg IV. Monitoring of patient was continued in the postoperative period till 2 hours after subarachnoid block.
Statistical Analysis
The response time after dexmedetomidine or nalbuphine treatment for post spinal shivering was taken as the primary parameter for sample size calculation. Based on previous literature, the mean effect size of 150 seconds between response time of both the drugs with a standard deviation (SD) of 100 seconds was hypothesized. By using statistical power of 80% and a level of significance of 0.01, sample size was calculated as 37 patients in each group. To reduce the probability of dropout, we enrolled 40 patients in each group. Data were composed, tabulated and then analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous parameters were expressed as mean ± SD while categorical parameters were expressed as percentage. With regard to continuous variables, two tailed unpaired student’s
Results
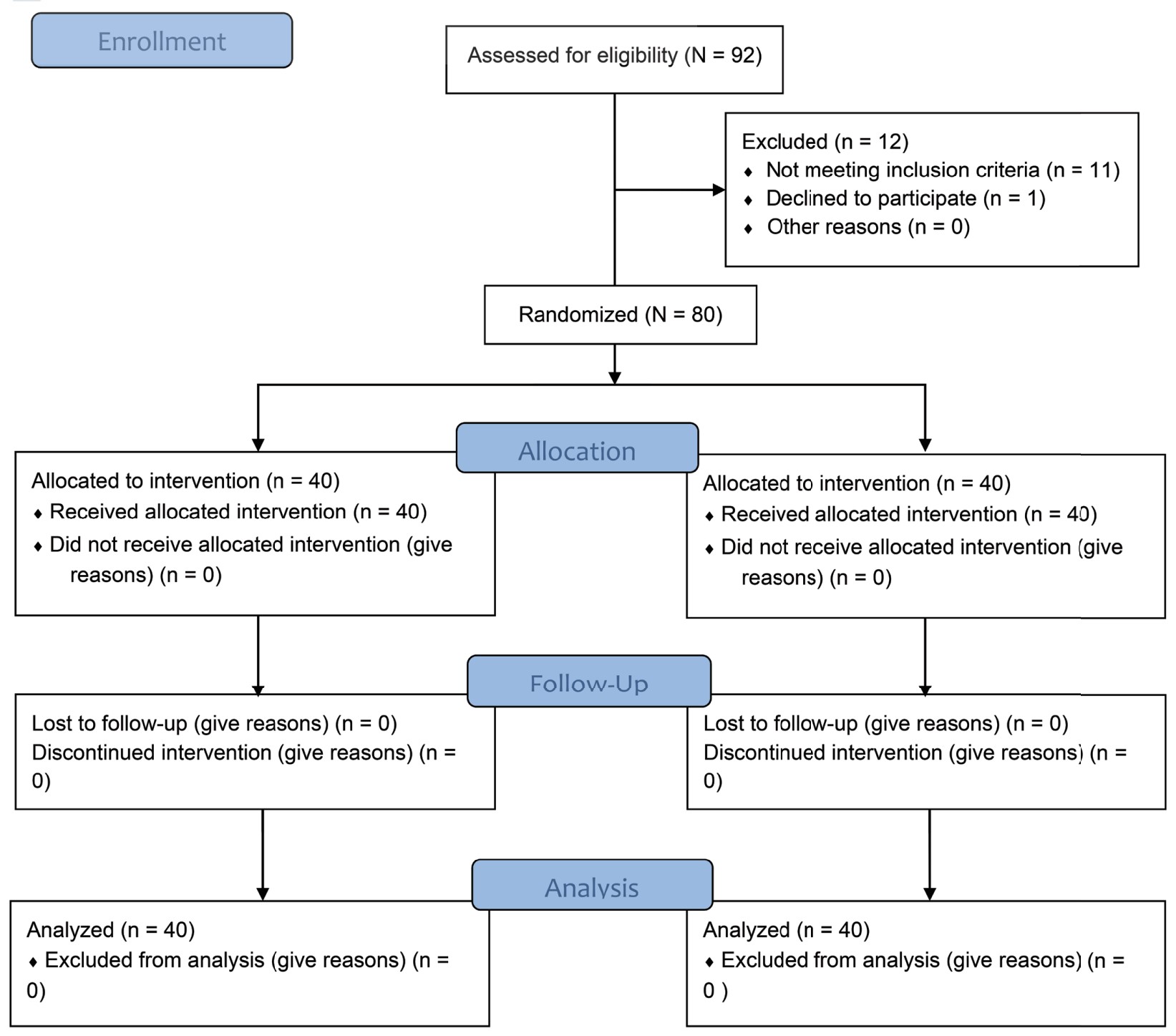
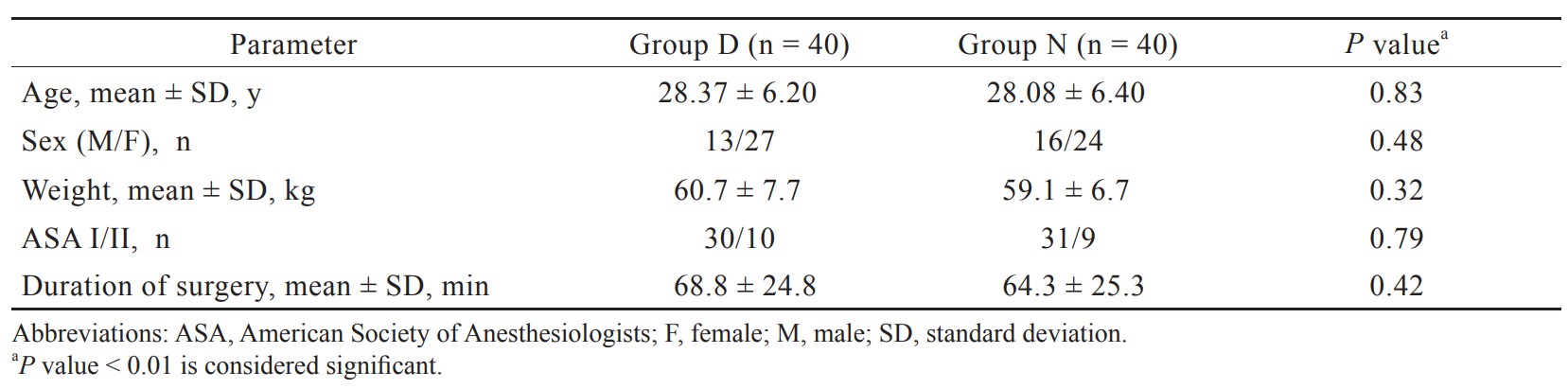
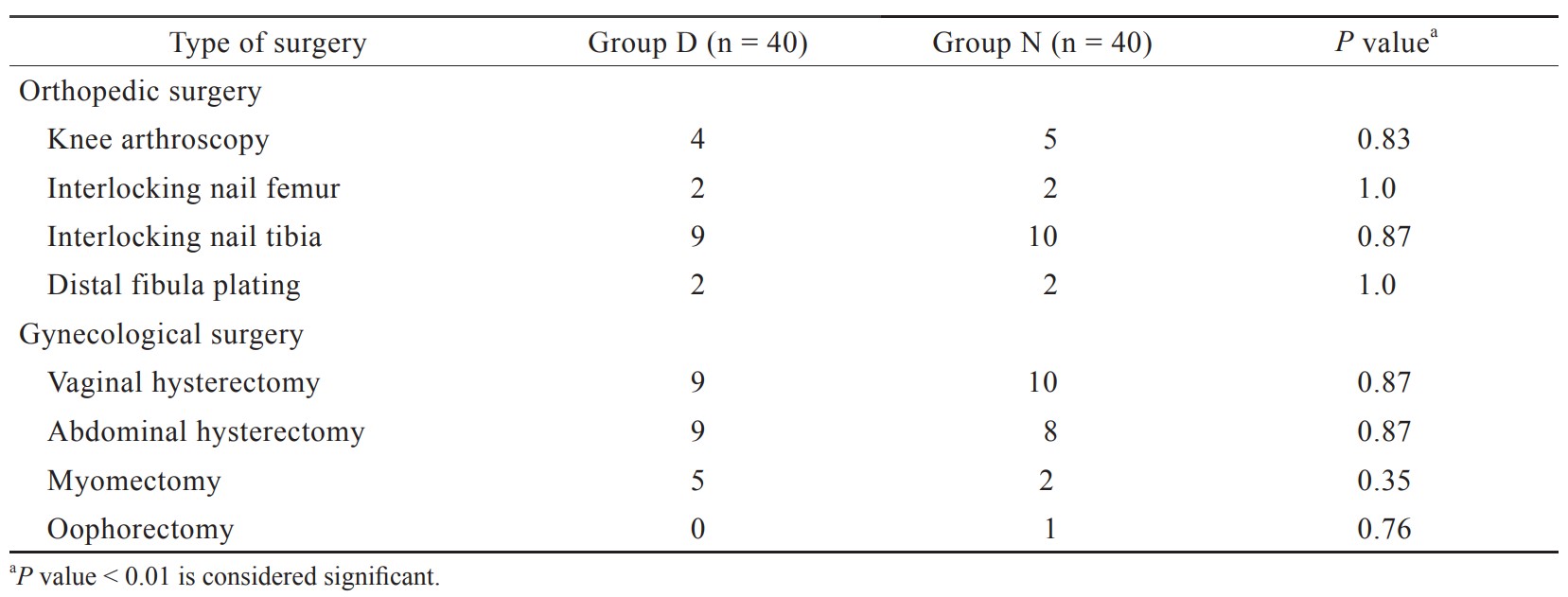
In the present study, total 92 patients were assessed, but 11 patients did not meet inclusion criteria (developing grades III or IV shivering), and 1 patient refused to participate (Figure 1). Therefore, total 80 patients had completed the trial successfully, and their data were analyzed as per protocol. Both the groups were comparable regarding demographic data including age, sex, weight, ASA grading, duration of surgery, and type of surgery (Tables 1 and 2). Baseline time of onset of shivering and grade of shivering were also comparable between these two groups (Table 3). In the present study, the mean response time was 1.9 ± 0.6 min in group D and 4.7 ± 1.1 min in group N, and the difference was statistically highly significant (
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image

Download full-size image
Download full-size image
Discussion
Shivering is a frequently occurring complication in patients undergoing spinal anesthesia. Following anesthesia, decline in core temperature occurs in three phases: (1) phase I —The highest decline happening at 30 minutes, (2) phase 2—Commences after 1 hour, and then (3) phase 3—Commences after 3–5 hours with reduced heat loss until equilibrium is reached.11 Core temperature falls because of sympathetic blockage, cold operating room, and rapid infusion of crystalloid solution at room temperature and direct effect of cold anesthesia solution on the thermosensitive structure of the spinal cord. We had planned this study to standardize a similar degree of these possible confounding factors between both the groups. The temperature of operating theatre was kept stable at 24–26°C, and drugs or IV fluids were used at room temperature in both the groups.
The core temperature monitoring sites for intraoperative hypothermia are tympanic membrane, pulmonary artery, distal esophagus and nasopharynx, which are not convenient for monitoring especially in conscious patient undergoing spinal anesthesia. Therefore, “near-core” sites like oral, axillary, and bladder, which are reasonably accurate for core temperature monitoring can be used. In the present study, we recorded only axillary temperature as it is relatively accurate.12 Kranke et al.13 concluded that use of prophylaxis for perioperative shivering is not cost effective. Therefore, we did a study for treatment instead of prophylaxis for shivering.
In our study, we used dexmedetomidine in doses of 0.5 μg/kg based on the results of a meta-analysis done by Liu et al.14 who found a minimum effective dose of dexmedetomidine as 0.5 μg/kg to manage postoperative shivering. Mittal et al.15 also used 0.5 μg/kg dexmedetomidine for post-spinal shivering treatment and found faster onset to control shivering. We used nalbuphine in a dose of 0.08 mg/kg for our study as Bhar et al.16 concluded that 0.08 mg/kg of nalbuphine is the best dose for shivering control with minimal adverse effects and increasing the doses further will only increase the possibility of complications. Other study had also used nalbuphine in a dose of 0.08 mg/kg with a rapid and potent antishivering effect.17
The present study found that the response time for treatment of shivering was much lesser (1.9 ± 0.6 min) in dexmedetomidine group as compared to (4.7 ± 1.1 min) nalbuphine group. Megalla and Mansour18 did a study with dexmedetomidine 0.5 μg/kg (group D) or nalbuphine 0.07 mg/kg (group N) or saline (group C) as treatment of shivering on 75 patients and found significantly lesser response time for treatment of shivering in group D compared to group N and group C (2.0 ± 0.6 min, 3.6 ± 0.8 min, and 12.4 ± 3.7 min, respectively) which were in concordance with our results. In another study done by Abdel-Ghaffar et al.19 who used meperidine 0.4 mg/kg (meperidine group), and dexmedetomidine 0.5 μg/kg (DEX I group), 0.3 μg/kg (DEX II group), or 0.2 μg/kg (DEX III group) for treatment of shivering after subarachnoid block, had found that the time taken for complete cessation of shivering was 96.9 ± 40.7 seconds in DEX I group which was compared to our results.
The mean response time of nalbuphine group in our study was in concordance with the Nirala et al.20 study, where time taken for the disappearance of shivering in nalbuphine group was 3.8 ± 1.2 min. Similarly, other study which used 0.1 mg/kg nalbuphine for treatment of shivering had found the response time of 3.8 ± 0.4 min which is similar to our results.21 In the study conducted by Bhar et al.,16 they found shorter response time (2.8 ± 0.5 min) for cessation of shivering at similar dose of nalbuphine (0.08 mg/kg), and this difference in the time may be due to higher grade of shivering in our study.
Success rate with both the drugs was 100% and compared with our study. Similarly, Panneer et al.22 who compared dexmedetomidine and clonidine in controlling post spinal shivering, had found 100% success rate in dexmedetomidine group (0.5 μg/kg). The success rate of nalbuphine group of our study was compared to 99% success rate with 0.08 mg/kg of nalbuphine in the study by Bhar et al.16 and 95% success rate with 0.07 mg/kg of nalbuphine in the study by Sun et al.23 Recurrence rates with dexmedetomidine in the range of 0%–10% while with nalbuphine in the range of 8%–15% had been reported by various studies which were in concordance with our findings.18,24,25
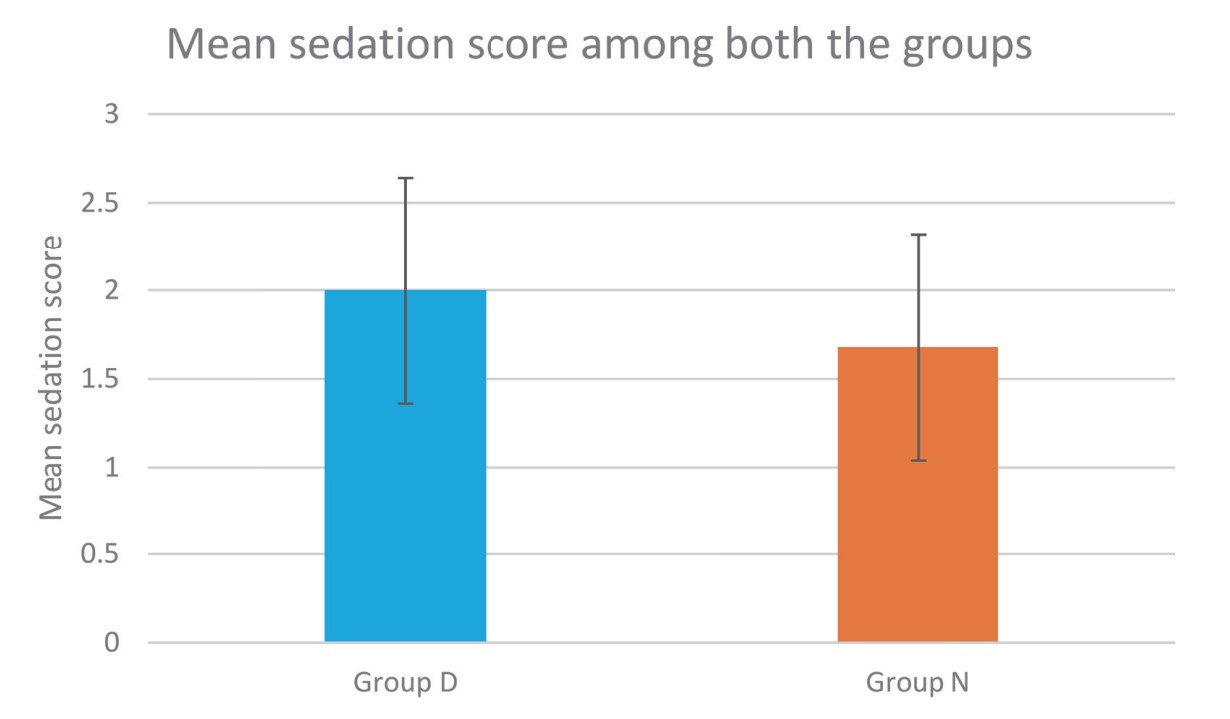
Sedation profile of both the groups in our study was compared using Filos sedation scale. Dexmedetomidine caused grade II sedation in 60% of patients without any respiratory depression which was more than nalbuphine group. Therefore, sedation in dexmedetomidine group provided additional comfort to the patients without any unwanted side effects similar to other studies.15,26
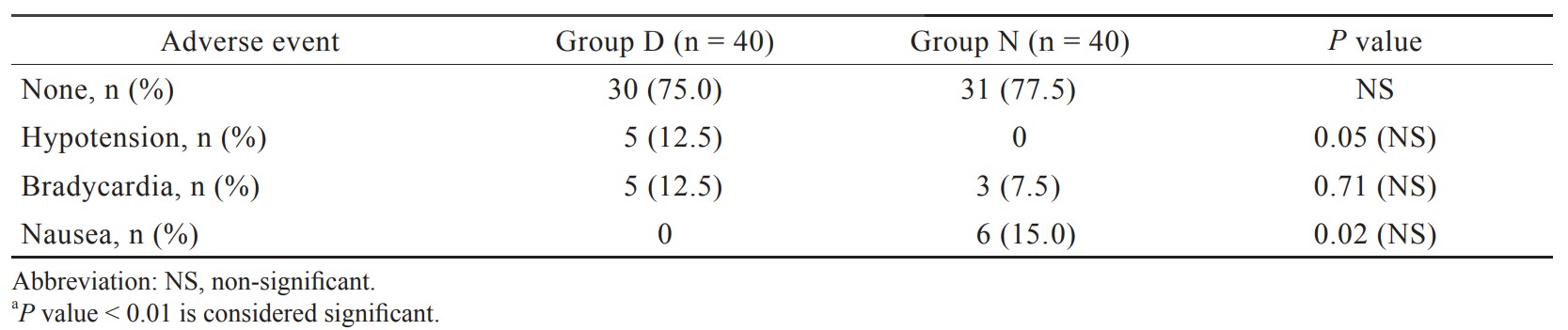
In our study, incidence of hypotension and bradycardia was observed in 12.5% of patients in group D which was statistically insignificant, and it was supported by the study done by Sun et al.23 and Wang et al.25 Though dexmedetomidine 0.5 μg/kg caused more hypotension and bradycardia compared to nalbuphine group, but it had not caused any significant harm to any patient in the present study. In our study, 15% incidence of nausea in the nalbuphine group was found similar to study done by Megalla and Mansour.18
Our study has some limitations. First, in our study, we could not measure the core body temperature as the sites like esophagus and tympanic membrane, were uncomfortable and unacceptable to the patients undergoing spinal anesthesia. Secondly, subjective observation index for grading of shivering was used in our study because of lack of reliable and objective methods for determining shivering. Thirdly, our study was restricted mainly to the intraoperative period without any prolonged postoperative follow-up for shivering. Fourth, the result of the present trial is limited by its integral elements and the cogency of its outcomes could not be appropriate in other types of anesthesia.
Conclusions
We conclude that both dexmedetomidine (0.50 μg/kg) and nalbuphine (0.08 mg/kg) can be used for treatment of post spinal shivering with limited drug related side effects (hypotension and bradycardia with dexmedetomidine use and nausea with nalbuphine use), but dexmedetomidine is a better choice as compared to nalbuphine because of faster response time and lesser recurrence rate. Furthermore, sedation caused by dexmedetomidine provides additional comforts to the patients.
References
| 1 |
De Witte J, Sessler DI.
Perioperative shivering: physiology and pharmacology.
Anesthesiology. 2002;96(2):467-484.
|
| 2 |
Shukla U, Malhotra K, Prabhakar T.
A comparative study of the effect of clonidine and tramadol on post-spinal anaesthesia shivering.
Indian J Anaesth. 2011;55(3):242-246.
|
| 3 |
Crowley LJ, Buggy DJ.
Shivering and neuraxial anesthesia.
Reg Anesth Pain Med. 2008;33(3):241-252.
|
| 4 |
Katyal S, Tewari A, Narula N.
Shivering: anaesthetic considerations.
J Anaesth Clin Pharmacol. 2002;18(4):363-376.
|
| 5 |
Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C.
Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds.
Anesthesiology. 1997;87(4):835-841.
|
| 6 |
Bansal P, Jain G.
Control of shivering with clonidine, butorphanol, and tramadol under spinal anesthesia: a comparative study.
Local Reg Anesth. 2011;4:29-34.
|
| 7 |
Ikeda T, Kurz A, Sessler DI, et al.
The effect of opioids on thermoregulatory responses in humans and the special antishivering action of meperidine.
Ann N Y Acad Sci. 1997;813:792-798.
|
| 8 |
Takada K, Clark DJ, Davies MF, et al.
Meperidine exerts agonist activity at the alpha(2B)-adrenoceptor subtype.
Anesthesiology. 2002;96(6):1420-1426.
|
| 9 |
Wrench IJ, Singh P, Dennis AR, Mahajan RP, Crossley AW.
The minimum effective doses of pethidine and doxapram in the treatment of post-anaesthetic shivering.
Anaesthesia. 1997;52(1):32-36.
|
| 10 |
Filos KS, Goudas LC, Patroni O, Polyzou V.
Hemodynamic and analgesic profile after intrathecal clonidine in humans.
Anesthesiology. 1994;81(3):591-601.
|
| 11 |
Sessler DI.
Temperature monitoring.
|
| 12 |
Lodha R, Mukerji N, Sinha N, Pandey RM, Jain Y.
Is axillary temperature an appropriate surrogate for core temperature.
Indian J Pediatr. 2000;67(8):571-574.
|
| 13 |
Kranke P, Eberhart LH, Roewer N, Tramèr MR.
Pharmacological treatment of postoperative shivering: a quantitative systematic review of randomized controlled trials.
Anesth Analg. 2002;94(2):453-460.
|
| 14 |
Liu ZX, Xu FY, Liang X, et al.
Efficacy of dexmedetomidine on postoperative shivering: a meta-analysis of clinical trials.
Can J Anaesth. 2015;62(7):816-829.
|
| 15 |
Mittal G, Gupta K, Katyal S, Kaushal S.
Randomised double-blind comparative study of dexmedetomidine and tramadol for post-spinal anaesthesia shivering.
Indian J Anaesth. 2014;58(3):257-262.
|
| 16 |
Bhar D, Basunia SR, Das A, et al.
Comparison between different doses of nalbuphine for management of post spinal shivering—a prospective, randomised, double blind study.
J Evolution Med Dent Sci. 2016;5(101):7438-7443.
|
| 17 |
Liu J, Huang S, Sun S, Sun X, Wang T.
Comparison of nalbuphine, ondansetron and placebo for the prevention of shivering after spinal anaesthesia for urgent caesarean delivery: a randomised double-blind controlled clinical trial.
Int J Obstet Anesth. 2020;42:39-46.
|
| 18 |
Megalla SA, Mansour HS.
Dexmedetomidine versus nalbuphine for treatment of postspinal shivering in patients undergoing vaginal hysterectomy: a randomized double blind, controlled study.
Egypt J Anaesth. 2017;33(1):47-52.
|
| 19 |
Abdel-Ghaffar HS, Mohamed SA, Fares KM, Osman MA.
Safety and efficacy of dexmedetomidine in treating post spinal anesthesia shivering: a randomized clinically controlled dose–finding trial.
Pain Physician. 2016;19(4):243-253.
|
| 20 |
Nirala DK, Prakash J, Ram B, Kumar V, Bhattacharya PK, Priye S.
Randomized double blinded comparative study of intravenous nalbuphine and tramadol for the treatment of postspinal anesthesia shivering.
Anesth Essays Res. 2020;14(3):510-514.
|
| 21 |
Vasanthy J, Senthil P.
A comparative study of effect of clonidine tramadol and nalbuphine on postspinal anaesthesia shivering.
Indian J Appl Res. 2018;8(5):52-55.
|
| 22 |
Panneer M, Murugaiyan P, Rao SV.
A comparative study of intravenous dexmedetomidine and intravenous clonidine for postspinal shivering in patients undergoing lower limb orthopedic surgeries.
Anesth Essays Res. 2017;11(1):151-154.
|
| 23 |
Sun J, Zheng Z, Li YL, et al.
Nalbuphine versus dexmedetomidine for treatment of combined spinal-epidural post-anesthetic shivering in pregnant women undergoing cesarean section.
J Int Med Res. 2019;47(9):4442-4453.
|
| 24 |
Verma A, Bhandari D, Dhande P, Jain S, Tidke S.
Comparative evaluation of dexmedetomidine and tramadol for attenuation of post-spinal anaesthesia shivering.
J Clin Diagn Res. 2018;12(6):UC01-UC04.
|
| 25 | |
| 26 |
Yu G, Jin S, Chen J, Yao W, Song X.
The effects of novel α2-adrenoreceptor agonist dexmedetomidine on shivering in patients underwent caesarean section.
Biosci Rep. 2019;39(2):BSR20181847.
|