Abstract
Liver transplantation (LT) is frequently complicated by coagulopathy associated with end-stage liver disease, which is often multifactorial and associated with hemostatic disturbances affecting both the procoagulant and anticoagulant systems. This rebalanced coagulation system may lead to bleeding diathesis or increased clot formation. Conventional coagulation tests cannot reflect these complex changes because they can only illustrate deficiencies in the procoagulant system. Viscoelastic tests such as rotational thromboelastometry (ROTEM) have been used in LT and have shown useful for detecting coagulopathy and guiding transfusions. Implementation of ROTEM-guided bleeding management algorithms has proven effectiveness in reducing bleeding, transfusion needs, complication rates, and healthcare costs in LT. This document is intended to provide a practice algorithm for the management of major bleeding and coagulopathy during LT and to encourage adaptation of the guidelines to individual institutional circumstances and resources.
Keywords
cirrhosis, hemostasis, liver transplantation, rotational thromboelastometry, viscoelastic testing
Introduction
Liver transplantation (LT) is often complicated by coagulopathy associated with end-stage liver disease (ESLD), surgical bleeding, and ischemia-reperfusion injury in new liver grafts.1,2 Among the factors contributing to bleeding in LT are the surgical technique and expertise of the surgeon, anatomical variations, ESLD severity, and renal impairment.3,4 Over the past decades, the requirement for transfusion during LT has become less common because of advancements in surgical techniques and coagulation monitoring and management.5 Standard laboratory coagulation tests (SLCTs), such as prothrombin time, the international normalized ratio (INR), activated partial thromboplastin time (aPTT), plasma fibrinogen, and platelet count, may not accurately predict bleeding in ESLD because these tests only measure the initiation of clotting; they do not consider clot strength, stability, and platelet function.2 ESLD coagulopathy involves an imbalance between procoagulant and anticoagulant factors, typically involving decreased levels of all coagulation factors except for factor VIII and Von Willebrand factor. Antithrombotic factors such as Proteins C and S are usually reduced. SLCTs such as INR and aPTT do not adequately reflect this hemostatic balance in patients because these tests typically measure the time required to initially generate thrombin in plasma as a function of procoagulant drivers and do not account for other cellular and biochemical factors involved in coagulation. Reductions in procoagulant and anticoagulant factors are often accompanied by qualitative and quantitative platelet defects, fibrinogen anomalies, and fibrinolytic dysregulation. Many studies have revealed a strong correlation between viscoelastic tests and in vivo bleeding and coagulation function.6,7 In addition, multiple clinicians have emphasized the importance of selecting appropriate treatments on the basis of point-of-care (POC) coagulation monitoring results and comprehensive data regarding allogeneic blood products, coagulation factor concentrations, and medication effects.8-10
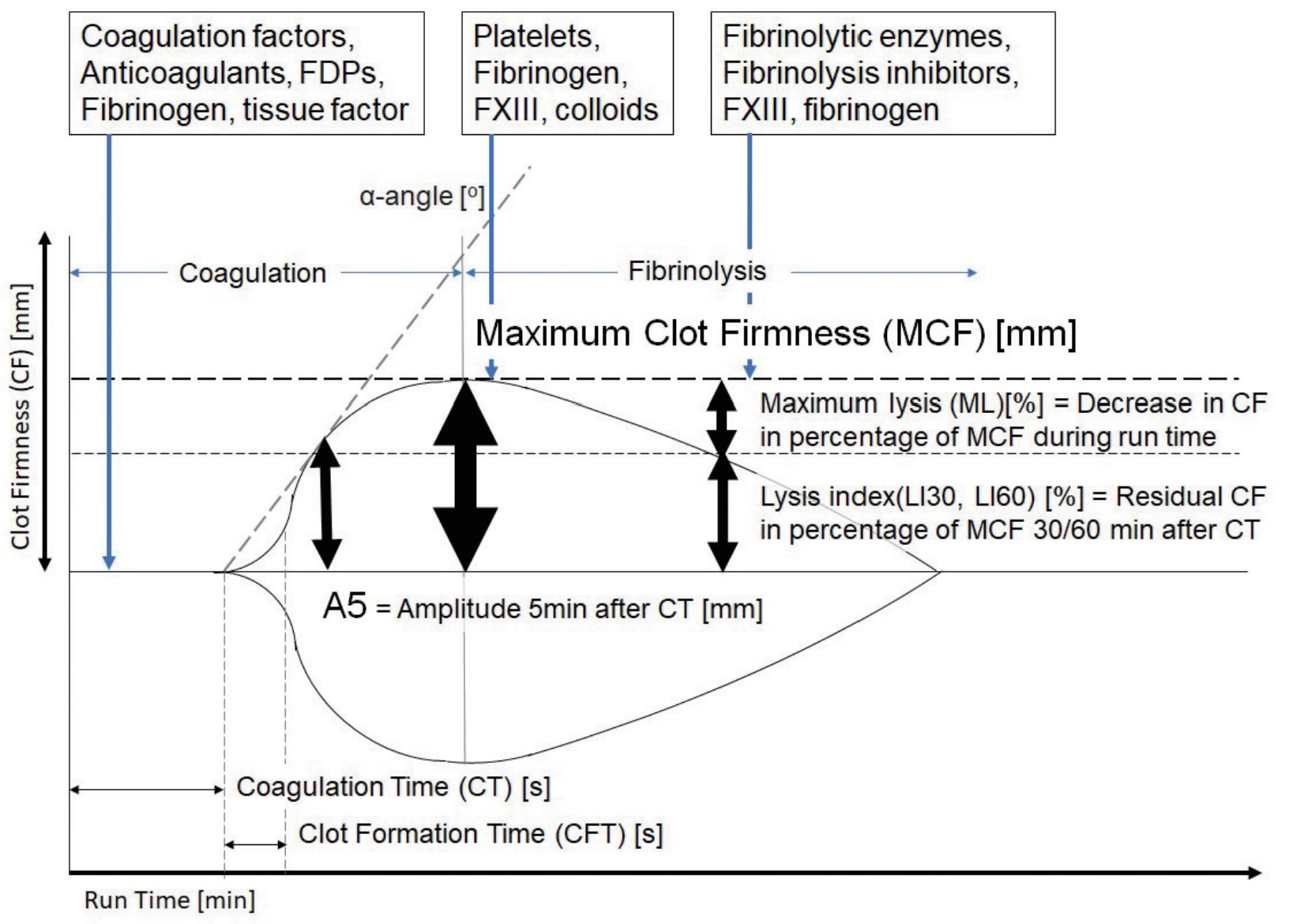
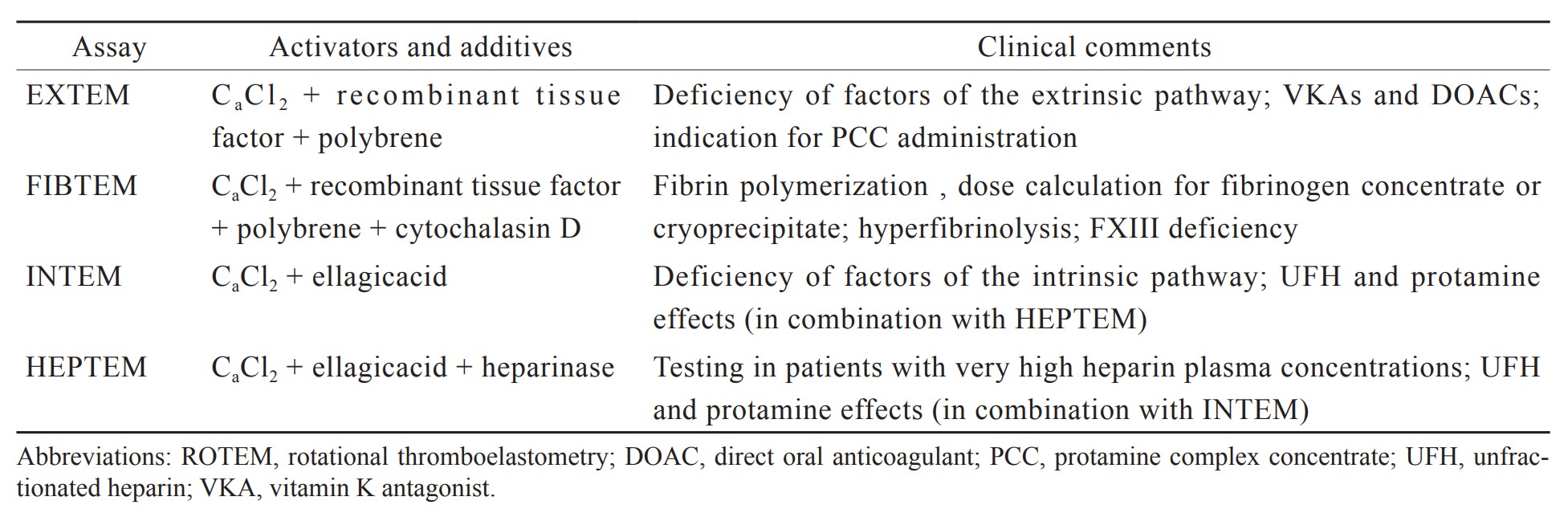
Bleeding management guided by rotational thromboelastometry (ROTEM) is a crucial aspect of patient blood management (PBM) that enhances patient safety.11 POC ROTEM can provide test results within 10–15 minutes, as indicated in Figure 1.12 Early amplitudes of clot firmness at 5 and 10 minutes (A5 and A10) after clot initiation (coagulation time) strongly correlate with maximum clot firmness (MCF), plasma fibrinogen concentration, and platelet count, which substantially reduces the turnaround time of ROTEM analysis (Table 1).13,14 Not only does POC ROTEM testing yield results faster than SLCTs do, but it also outperforms SLCTs in predicting bleeding and transfusion requirements during LT.15 Research has indicated that implementing ROTEM-guided bleeding management algorithms considerably reduces bleeding, transfusion requirements, complications, and hospital expenses for LT.16-26 We established a practice algorithm for ROTEM-guided bleeding management for trauma and orthopedic surgery in Taiwan.12 This clinical management strategy has the potential to ensure a uniform standard of care within Taiwan and, potentially, other countries and can therefore improve outcomes for patients undergoing LT.
Download full-size image
Download full-size image
Evidence-Based ROTEM-Guided Algorithm in Liver Transplantation
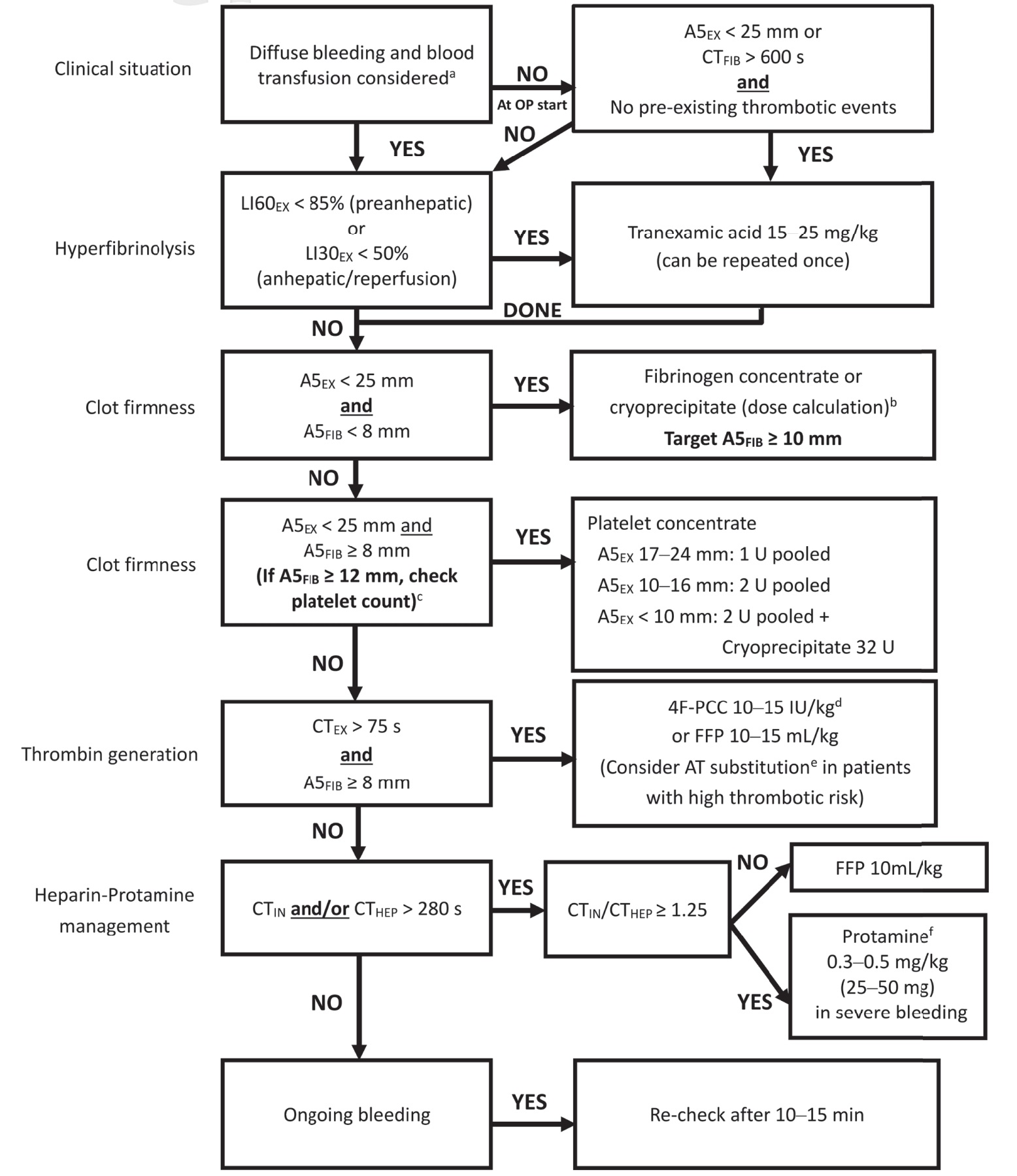
As presented in Figure 2, the ROTEM A5 algorithm for LT starts with clinical identification of diffuse bleeding and blood transfusion, followed by management of fibrinolysis, clot firmness, and thrombin generation. In patients with cirrhosis, SLCTs are often associated with thrombocytopenia and an elevated INR. However, these pathological conditions are not associated with an increased risk of bleeding, and therefore, reconsideration of hemostatic balance is required for this patient population. Massive blood transfusions are associated with nosocomial infections, citrate toxicity, transfusion-related acute lung injury, transfusion-associated circulatory overload (TACO), and portal hypertension, which may exacerbate bleeding and lead to higher hospital mortality rates.27,28 Adopting a restrictive transfusion approach has been associated with decreased mortality in patients with cirrhosis and upper gastrointestinal bleeding.29
Download full-size image
aTiming of ROTEM-analysis during liver transplantation: Baseline; re-check after 60 min or in case of bleeding during pre-anhepatic phase; 10 min after cava clamping; and always in case of diffuse bleeding as well as 10–15 min after a specific hemostatic intervention.
Fibrinogen deficiency often results in prolonged clotting time in the EXTEM assay (CTEX). For CTEX values to be interpretable, an adequate early fibrinogen clot amplitude (FIBTEM) must be obtained within the first 5 minutes (A5FIB). For ROTEM, results should be analyzed sequentially as outlined in the algorithm, with prioritization of A5FIB over CTEX. This sequence reflects the physiological process in which fibrinogen levels initially decrease during severe bleeding and subsequently affect thrombin generation.
Management of fibrinolysis and detection of endogenous heparin-like effects (HLEs) are essential in LT. Fibrinolysis occurs in 60%–80% of patients undergoing LT, most commonly after reperfusion. However, these events typically become self-limiting within 30–180 minutes and do not require additional intervention.30,31 Fibrinolysis during liver resection is associated with increased mortality at 30 days and 6 months, and postreperfusion fibrinolysis is associated with portal vein and hepatic artery thrombosis.31 Clinicians should carefully consider whether antifibrinolytic drugs should be administered, particularly when fibrinolysis occurs after reperfusion.30,31 Low clot firmness (A5EX < 25 mm) in EXTEM and a flat line (CTFIB > 600 s) in FIBTEM are reliable predictors of fibrinolysis and can guide risk assessment at the onset of surgery.32,33 FIBTEM is the most sensitive assay for detecting fibrinolysis because its diagnostic accuracy is not influenced by platelet-mediated clot retraction.34,35
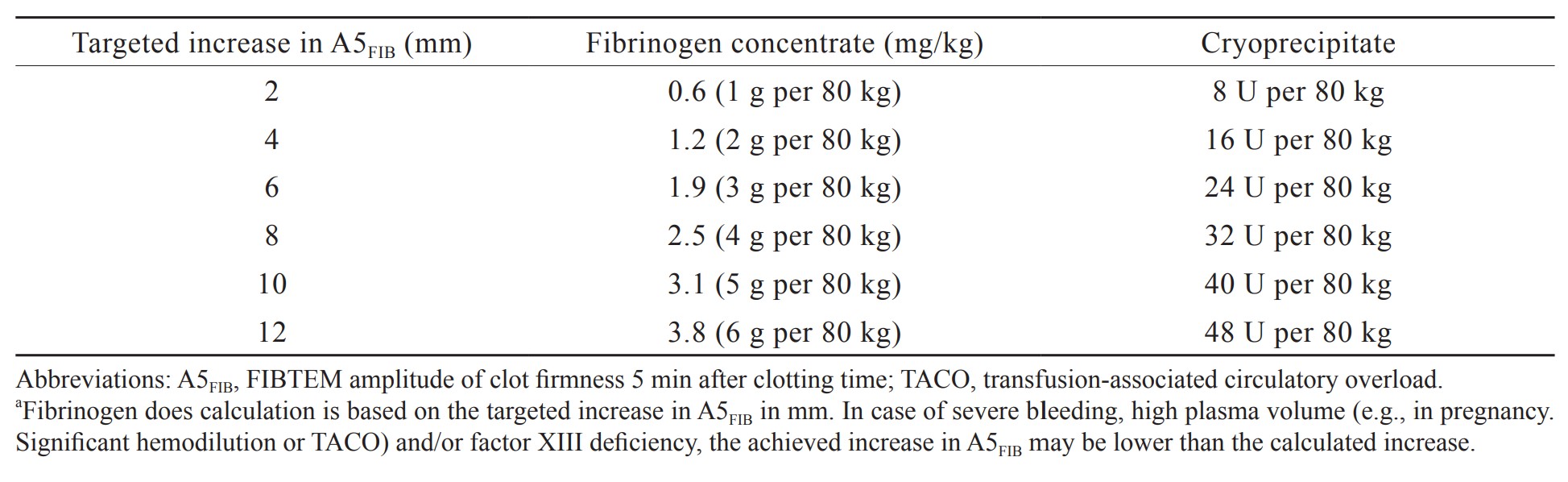
Research has identified specific cutoff values for EXTEM and FIBTEM clot firmness amplitudes (A5, A10, and MCF) that can predict bleeding and inform decisions regarding fibrinogen replacement and platelet transfusion during and after LT.15,36-38 When cutoff values are set at 25 mm for A5EX and 8 mm for A5FIB, lower clot firmness seems to be acceptable for LT. Notably, FIBTEM is a more effective predictive value for hemorrhage in LT than plasma fibrinogen levels because it can be used to evaluate not only the quantity of fibrinogen but also fibrin polymerization, which can be influenced by dysfibrinogenemia, factor XIII activity, and colloids.39-41 Fibrinogen is calculated on the basis of the target increase in A5FIB (in millimeters) amplitude, augmented by a fibrinogen concentrate or cryoprecipitate (Table 2). The adoption of FIBTEM-guided fibrinogen replacement strategies in bleeding management algorithms has notably reduced the need for transfusions of red blood cells, plasma, and platelets during LT.15,17-21,42 Nevertheless, the prophylactic use of fibrinogen concentrates has not affected the need for transfusion in randomized controlled trials of LT.43
Download full-size image
In scenarios where platelet counts are below 50 × 109/L without accompanying bleeding events, ROTEM-guided platelet transfusion during LT (in patients with cirrhosis undergoing invasive procedures) has been found to reduce the need for platelet transfusion by 64%–75%.42,44-46 This finding is particularly notable because platelet transfusion during LT is associated with a decreased 1-year survival rate (74% vs. 92%).47
The INR can be used to gauge the severity of liver disease but cannot be used for evaluating thrombin generation and hemorrhage risk in patients with cirrhosis.48-50 CTEX with a cutoff of 75 seconds is useful for predicting bleeding in this patient population, and CTEX guidance can considerably reduce the need for fresh frozen plasma (FFP) and prothrombin complex concentrate (PCC) transfusions, which can mitigate the risk of overtreatment and thromboembolic events.15,42,48-53 FFP is not effective in increasing thrombin generation in patients with cirrhosis and is associated with an increased risk of TACO and portal hypertension.19,42,49,53
Endogenous heparinization or HLEs has been reported to occur in patients undergoing LT.32,54,55 In approximately 50% of LT cases, mild (CTIN/CTHEP ratio ≥ 1.25) to severe (CTIN/CTHEP ratio ≥ 2.0) HLEs are detected after reperfusion. Compared with aPTT, the CTIN/CTHEP ratio is more sensitive as an indicator of HLEs. Severe HLEs are associated with a greater need for transfusion, and their presence during the anhepatic phase is associated with increased 3-month mortality.56 Postreperfusion HLEs are typically self-limited after hemodynamic stabilization. If not, they can be reversed with a small dose of protamine.57
ROTEM results can be used to assist risk assessment for thrombosis and can thereby aid in the prevention of thromboembolic complications.15,19,21 Studies on patients with cirrhosis and LT recipients have indicated that elevated MCFFIB with cutoff values between 18 and 25 mm (risk ratio of up to 4.8) predicts hepatic artery and portal vein thrombosis.58-60
There is relevant literature to guide the time points for initiating ROTEM analyses.61,62 However, these measurements at specific points in time are only recommended and not required. The most important thing is to measure the presence of bleeding and to check the effects of the intervention. As thromboembolic complications such as portal vein and hepatic artery thrombosis are of increasing concern, postoperative measurement at the ICU and/or before discharge from the hospital has become even more important. It depends on the major problems (bleeding, thrombosis, both) in liver transplant recipients in the individual hospital.
Effects of Implementing a ROTEM-Guided Bleeding Management Algorithm on Immediate and Short-Term LT Outcomes
Transfusion Requirements
Multiple studies have indicated that implementing a ROTEM-guided transfusion algorithm reduces the need for allogeneic blood products (red blood cells, platelets, and FFP).18,20-26 However, other studies have indicated that coagulation monitoring with ROTEM is associated with an increased use of concentrated factors (fibrinogen concentrate and PCC), even with reduced allogeneic product use.20-22,25,26,63,64
Postoperative Complications
Three studies investigating postoperative complications revealed major benefits from employing a ROTEM-guided algorithm. These benefits include lower rates of reoperation for bleeding (13% vs. 5%), retransplantation (10% vs. 2%), acute kidney injury (17% vs. 2%), and overall hemodynamic instability (29% vs. 16%).20,23,25 However, they also reported a high incidence of neurological complications (14% vs. 27%) and postoperative viral infections (7% vs. 20%) in the ROTEM group, although these findings may be attributable to the inherent risks of multiple comparisons.20 Another study involving a cohort of 336 patients undergoing LT reported significant reductions in the rates of reoperation for bleeding (8.3% vs. 2.4%), acute kidney injury (33.6% vs. 5.4%), and postoperative bleeding (8.7% vs. 3.6%) with the adoption of a ROTEM-guided transfusion algorithm relative to the use of conventional coagulation tests (CCTs).23
Length of Hospital Stay
One study reported that patients who were managed using a ROTEM-based transfusion algorithm experienced a 2-day reduction in hospital length of stay (LOS) relative to that of those managed using CCTs (40.6 vs. 38.2 days), which was due to a reduction in intensive care unit (ICU) LOS (10.2 vs. 8.4 days).23 By contrast, four studies did not report a reduction in LOS for patients treated with ROTEM relative to that of those treated with CCTs.21,22,24,63 Currently, evidence supporting the routine use of ROTEM-based transfusion management for the purpose of reducing hospital or ICU LOS is insufficient.
Cost-Effectiveness
Two studies investigating cost-effectiveness reported overall cost savings for patients managed using a ROTEM-guided transfusion algorithm.22,23 Although the intraoperative use of ROTEM was generally more expensive than that of CCTs in a direct comparison of testing costs, evidence indicated that ROTEM led to major cost savings because it was associated with reduced consumption of allogeneic blood products and fewer transfusion-related complications.
Survival
Eight studies explored the effects of using ROTEM on mortality or survival, investigating the effects from immediate ICU mortality to 3-year overall survival. None of the studies identified a survival advantage for ROTEM over CCTs.18,20-25,63
Conclusion
Overall, our findings highlight the importance of a practice algorithm for ROTEM-guided bleeding management for accurate dose adjustment, and they emphasize that overtreatment must be strictly avoided. This clinical practice guideline can be used to establish a uniform standard of care in Taiwan and enhance outcomes for LT recipients through education and integration into local practice.
References
| 1 |
Kang Y, Audu P.
Coagulation and liver transplantation.
Int Anesthesiol Clin. 2006;44(4):17-36.
|
| 2 |
Forkin KT, Colquhoun DA, Nemergut EC, Huffmyer JL.
The coagulation profile of end-stage liver disease and considerations for intraoperative management.
Anesth Analg. 2018;126(1):46-61.
|
| 3 |
Biancofiore G, Blasi A, De Boer MT, et al.
Perioperative hemostatic management in the cirrhotic patient: a position paper on behalf of the Liver Intensive Care Group of Europe (LICAGE).
Minerva Anestesiol. 2019;85(7):782-798.
|
| 4 |
Clevenger B, Mallett SV.
Transfusion and coagulation management in liver transplantation.
World J Gastroenterol. 2014;20(20):6146-6158.
|
| 5 |
Kang Y.
Coagulation and liver transplantation: current concepts.
Liver Transplant Surg. 1997;3(4):465-467.
|
| 6 |
Yoon U, Lai M, Nguyen T, Elia E.
Perioperative viscoelastic assay use for monitoring coagulation among us liver transplantation centers.
Transplant Proc. 2021;53(7):2312-2317.
|
| 7 |
Sakai T.
Viscoelastic testing in liver transplantation.
Transfusion. 2020;60(Suppl 6):S61-S69.
|
| 8 |
Bezinover D, Dirkmann D, Findlay J, et al.
Perioperative coagulation management in liver transplant recipients.
Transplantation. 2018;102(4):578-592.
|
| 9 |
Buliarca A, Horhat A, Mocan T, Craciun R, Procopet B, Sparchez Z.
Viscoelastic tests in liver disease: where do we stand now?
World J Gastroenterol. 2021;27(23):3290-3302.
|
| 10 |
Wei H, Child LJ.
Clinical utility of viscoelastic testing in chronic liver disease: a systematic review.
World J Hepatol. 2020;12(11):1115-1127.
|
| 11 |
Zacharowski K, Spahn DR.
Patient blood management equals patient safety.
Best Pract Res Clin Anaesthesiol. 2016;30(2):159-169.
|
| 12 |
Zheng ZH, Yeh TT, Yeh CC, Lu CH.
Practice algorithm of rotational thromboelastometry-guided bleeding management in trauma and orthopedic surgery.
J Med Sci. 2022;42(2):57-63.
|
| 13 |
Görlinger K, Dirkmann D, Solomon C, Hanke AA.
Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability.
Br J Anaesth. 2013;110(2):222-230.
|
| 14 |
Song JG, Jeong SM, Jun IG, Lee HM, Hwang GS.
Five-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation.
Br J Anaesth. 2014;112(2):290-297.
|
| 15 |
Dötsch TM, Dirkmann D, Bezinover D, et al.
Assessment of standard laboratory tests and rotational thromboelastometry for the prediction of postoperative bleeding in liver transplantation.
Br J Anaesth. 2017;119(3):402-410.
|
| 16 |
Wikkelsø A, Wetterslev J, Møller AM, Afshari A.
Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding.
Cochrane Database Syst Rev. 2016;2016(8):CD007871.
|
| 17 |
Noval-Padillo JA, León-Justel A, Mellado-Miras P, et al.
Introduction of fibrinogen in the treatment of hemostatic disorders during orthotopic liver transplantation: implications in the use of allogenic blood.
Transplant Proc. 2010;42(8):2973-2974.
|
| 18 |
Alamo JM, León A, Mellado P, et al.
Is “intra-operating room” thromboelastometry useful in liver transplantation?
Transplant Proc. 2013;45(10):3637-3639.
|
| 19 |
Kirchner C, Dirkmann D, Treckmann JW, et al.
Coagulation management with factor concentrates in liver transplantation: a single-center experience.
Transfusion. 2014;54(10 Pt 2):2760-2768.
|
| 20 |
Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, et al.
Point-of-care haemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome.
Clin Chim Acta. 2015;446:277-283.
|
| 21 |
Zamper RPC, Amorim TC, Queiroz VN, et al.
Association between viscoelastic tests-guided therapy with synthetic factor concentrates and allogenic blood transfusion in liver transplantation: a before-after study.
BMC Anesthesiol. 2018;18(1):198.
|
| 22 |
Smart L, Mumtaz K, Scharpf D, et al.
Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost.
Ann Hepatol. 2017;16(6):916-923.
|
| 23 |
Leon-Justel A, Alvarez-Rios AI, Noval-Padillo JA, et al.
Point-of-care haemostasis monitoring during liver transplantation is cost effective.
Clin Chem Lab Med. 2019;57(6):883-890.
|
| 24 |
Schumacher C, Eismann H, Sieg L, et al.
Use of rotational thromboelastometry in liver transplantation is associated with reduced transfusion requirements.
Exp Clin Transplant. 2019;17(2):222-230.
|
| 25 |
Bonnet A, Gilquin N, Steer N, et al.
The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study.
Eur J Anaesthesiol. 2019;36(11):825-833.
|
| 26 |
Roullet S, Freyburger G, Cruc M, et al.
Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry-based algorithm.
Liver Transpl. 2015;21(2):169-179.
|
| 27 |
Smith NK, Kim S, Hill B, Goldberg A, DeMaria S, Zerillo J.
Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) in liver transplantation: a case report and focused review.
Semin Cardiothorac Vasc Anesth. 2018;22(2):180-190.
|
| 28 |
Pandey CK, Singh A, Kajal K, et al.
Intraoperative blood loss in orthotopic liver transplantation: the predictive factors.
World J Gastrointest Surg. 2015;7(6):86-93.
|
| 29 |
Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS.
Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials.
World J Gastroenterol. 2013;19(40):6919-6927.
|
| 30 |
Poon KS, Chen CC, Thorat A, et al.
Fibrinolysis after reperfusion of liver graft.
Acta Anaesthesiol Taiwan. 2015;53(1):41-43.
|
| 31 |
Shimauchi T, Yamaura K, Higashi M, Abe K, Yoshizumi T, Hoka S.
Fibrinolysis in living donor liver transplantation recipients evaluated using thromboelastometry: impact on mortality.
Transplant Proc. 2017;49(9):2117-2121.
|
| 32 |
Dirkmann D, Görlinger K, Peters J.
Assessment of early thromboelastometric variables from extrinsically activated assays with and without aprotinin for rapid detection of fibrinolysis.
Anesth Analg. 2014;119(3):533-542.
|
| 33 |
Kim EH, Song SH, Kim GS, Ko JS, Gwak MS, Lee SK.
Evaluation of “flat-line” thromboelastography after reperfusion during liver transplantation.
Transplant Proc. 2015;47(2):457-459.
|
| 34 |
Abuelkasem E, Lu S, Tanaka K, Planinsic R, Sakai T.
Comparison between thrombelastography and thromboelastometry in hyperfibrinolysis detection during adult liver transplantation.
Br J Anaesth. 2016;116(4):507-512.
|
| 35 |
Katori N, Tanaka KA, Szlam F, Levy JH.
The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography.
Anesth Analg. 2005;100(6):1781-1785.
|
| 36 |
Blasi A, Beltran J, Pereira A, et al.
An assessment of thromboelastometry to monitor blood coagulation and guide transfusion support in liver transplantation.
Transfusion. 2012;52(9):1989-1998.
|
| 37 |
Fayed N, Mourad W, Yassen K, Görlinger K.
Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation.
Transfus Med Hemother. 2015;42(2):99-108.
|
| 38 |
Sabate A, Blasi A, Costa M, Reyes R, Beltran J, Torres F.
Assessment of rotational thromboelastometry for the prediction of red blood cell requirements in orthotopic liver transplantation.
Minerva Anestesiol. 2018;84(4):447-454.
|
| 39 |
Bedreli S, Sowa JP, Malek S, et al.
Rotational thromboelastometry can detect factor XIII deficiency and bleeding diathesis in patients with cirrhosis.
Liver Int. 2017;37(4):562-568.
|
| 40 |
Raspé C, Besch M, Charitos EI, et al.
Rotational thromboelastometry for assessing bleeding complications and factor XIII deficiency in cardiac surgery patients.
Clin Appl Thromb Hemost. 2018;24(9_suppl):136S-144S.
|
| 41 |
Fenger-Eriksen C, Moore GW, Rangarajan S, Ingerslev J, Sørensen B.
Fibrinogen estimates are influenced by methods of measurement and hemodilution with colloid plasma expanders.
Transfusion. 2010;50(12):2571-2576.
|
| 42 |
Görlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schöchl H.
Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy.
Transfus Med Hemother. 2012;39(2):104-113.
|
| 43 |
Sabate A, Gutierrez R, Beltran J, et al.
Impact of preemptive fibrinogen concentrate on transfusion requirements in liver transplantation: a multicenter, randomized, double-blind, placebo-controlled trial.
Am J Transplant. 2016;16(8):2421-2429.
|
| 44 |
Fayed NA, Abdallah AR, Khalil MK, Marwan IK.
Therapeutic rather than prophylactic platelet transfusion policy for severe thrombocytopenia during liver transplantation.
Platelets. 2014;25(8):576-586.
|
| 45 |
Debernardi Venon W, Ponzo P, Sacco M, et al.
Usefulness of thromboelastometry in predicting the risk of bleeding in cirrhotics who undergo invasive procedures.
Eur J Gastroenterol Hepatol. 2015;27(11):1313-1319.
|
| 46 |
Basili S, Raparelli V, Napoleone L, et al.
Platelet count does not predict bleeding in cirrhotic patients: results from the PRO-LIVER study.
Am J Gastroenterol. 2018;113(3):368-375.
|
| 47 |
Pereboom ITA, de Boer MT, Haagsma EB, Hendriks HGD, Lisman T, Porte RJ.
Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury.
Anesth Analg. 2009;108(4):1083-1091.
|
| 48 |
Mallett SV, Sugavanam A, Krzanicki DA, et al.
Alterations in coagulation following major liver resection.
Anaesthesia. 2016;71(6):657-668.
|
| 49 |
Saner FH, Kirchner C.
Monitoring and treatment of coagulation disorders in end-stage liver disease.
Visc Med. 2016;32(4):241-248.
|
| 50 |
Tripodi A, Primignani M, Mannucci PM, Caldwell SH.
Changing concepts of cirrhotic coagulopathy.
Am J Gastroenterol. 2017;112(2):274-281.
|
| 51 |
Abuelkasem E, Mazzeffi MA, Lu SY, Planinsic RM, Sakai T, Tanaka KA.
Ex vivo evaluation of 4 different viscoelastic assays for detecting moderate to severe coagulopathy during liver transplantation.
Liver Transpl. 2016;22(4):468-475.
|
| 52 |
Bedreli S, Sowa JP, Gerken G, Saner FH, Canbay A.
Management of acute-on-chronic liver failure: rotational thromboelastometry may reduce substitution of coagulation factors in liver cirrhosis.
Gut. 2016;65(2):357-358.
|
| 53 |
Abuelkasem E, Hasan S, Mazzeffi MA, Planinsic RM, Sakai T, Tanaka KA.
Reduced requirement for prothrombin complex concentrate for the restoration of thrombin generation in plasma from liver transplant recipients.
Anesth Analg. 2017;125(2):609-615.
|
| 54 |
Kettner SC, Gonano C, Seebach F, et al.
Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation.
Anesth Analg. 1998;86(4):691-695.
|
| 55 |
Senzolo M, Agarwal S, Zappoli P, Vibhakorn S, Mallett S, Burroughs AK.
Heparin-like effect contributes to the coagulopathy in patients with acute liver failure undergoing liver transplantation.
Liver Int. 2009;29(5):754-759.
|
| 56 |
Yassen K, Refaat E, Helal S, Metwally A, Youssef S, Görlinger K.
Perioperative heparinase rotational thromboelastometry monitoring during and after adult living related liver transplantation.
Eur J Anaesthesiol. 2018;35(Suppl 56):286.
|
| 57 |
Gouvêa G, Toledo R, Diaz R, Auler L, Enne M, Martinho JM.
Protamine sulphate for treatment of severe post-reperfusion coagulopathy in pediatric liver transplantation.
Pediatr Transplant. 2009;13(8):1053-1057.
|
| 58 |
Rossetto V, Spiezia L, Senzolo M, Rodriguez-Castro KI, Maggiolo S, Simioni P.
Whole blood rotation thromboelastometry (ROTEM®) profiles in subjects with non-neoplastic portal vein thrombosis.
Thromb Res. 2013;132(2): e131-e134.
|
| 59 |
Zanetto A, Senzolo M, Vitale A, et al.
Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma.
Dig Liver Dis. 2017;49(4):440-445.
|
| 60 |
Kamel Y, Hassanin A, Ahmed AR, et al.
Perioperative thromboelastometry for adult living donor liver transplant recipients with a tendency to hypercoagulability: a prospective observational cohort study.
Transfus Med Hemother. 2018;45(6):404-412.
|
| 61 |
Görlinger K, Pérez-Ferrer A, Dirkmann D, et al.
The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management.
Korean J Anesthesiol. 2019;72(4):297-322.
|
| 62 |
Pérez-Calatayud AA, Hofmann A, Pérez-Ferrer A, et al.
Patient blood management in liver transplant—a concise review.
Biomedicines. 2023;11(4):1093.
|
| 63 |
Nascimento JCR, Neto EBL, da Silva EL, et al.
Analysis of the hemostatic therapy in liver transplantation guided by rotational thromboelastometry or conventional laboratory tests.
Eur J Gastroenterol Hepatol. 2020;32(11):1452-1457.
|
| 64 |
Schumann R, Mandell MS, Mercaldo N, et al.
Anesthesia for liver transplantation in United States academic centers: intraoperative practice.
J Clin Anesth. 2013;25(7):542-550.
|