Abstract
Elderly patients are more vulnerable to cognitive dysfunction in the postoperative period. Patients who are apparently well in cognitive functions in the preoperative period after undergoing anesthesia in noncardiac surgery will develop symptoms of cognitive dysfunction. Postoperative cognitive dysfunction (POCD) doesn’t continue for a long duration and usually undergoes self-resolution. Proper definitions and congruous tests for diagnosis are absent. Rigorous preoperative assessment of cognitive function and distinguishing risk factors are indispensable for recognizing the range of POCD and its association with surgery and anesthesia. Recent studies haven’t revealed any anesthesia technique or drug which can significantly reduce the incidence of POCD. Therefore, giving accurate information to patients can be challenging.
Keywords
cognitive disorders, delirium, amnesia, dementia, postoperative cognitive complications
Introuction
Postoperative cognitive dysfunction (POCD) was described as early as in 1955 as “adverse cerebral effects of anesthesia on old people”. 1 POCD is a clinical condition where patients experience a deterioration in cognitive function in the form of diminished memory, reduced attention span, and difficulty in decision-making, after surgery and anesthesia.
There are various forms of cognitive dysfunction which can occur in the postoperative period even among apparently well patients with no previous history of cognitive impairment. One of the most common and earliest forms of cognitive dysfunction is delirium which presents as an acute state of confusion associated with disturbance in attention and decreased awareness towards the environment. Severe symptoms of delirium require treatment as recommended by The National Institute for Health and Care Excellence guidelines.2
POCD is a relatively common complication of surgery, particularly in older patients or those undergoing more invasive procedures. The exact cause of POCD is not fully understood, but it is believed to be related to a combination of factors, including anesthesia & surgery-related inflammation, age-related factors, and underlying health conditions.
The majority of the research regarding POCD manifestations has been focused on cardiac surgery. Other conditions commonly associated with cognitive impairment include HIV/AIDS and Alzheimer’s disease. Regardless of the exact incidence, POCD can have significant impacts on patients’ quality of life and recovery from surgery. Patients with POCD may experience difficulties with daily activities, such as work or household tasks, and may require additional support and care. There is currently no definitive treatment for POCD, but some interventions may help to mitigate its effects. These include cognitive rehabilitation, physical exercise, and social engagement. Additionally, efforts to minimize the risk of POCD, such as careful monitoring of anesthesia and inflammation may be beneficial.
This review article aims to present the existing perception of POCD in non-cardiac surgery to proselytize integrative dialogue.
Definitions
Delirium is defined as a state of acute confusion presenting symptoms that may or may not be associated with organic illness.3 The
Postoperative delirium presents with an acute onset state of confusion and disorientation which varies throughout the day with periods of alternating hyperactivity, hypoactivity or mixed type usually associated with sleepiness in the day and agitation in the night,5,6 presenting most commonly within first 3 days after surgery.
Emergence delirium is differentiated from postoperative delirium on the basis of onset. Emergence delirium occurs immediately after anesthesia during the transition to awakening and is marked by agitation and hyperactivity.7,8 POCD and postoperative delirium increases the duration of hospital stay, associated costs and mortality.9,10
The major manifestation is significant cognitive decline as compared to a previous level, leading to interference with independence in daily activities. POCD can be diagnosed using a number of neuropsychological tests. (Table 1)
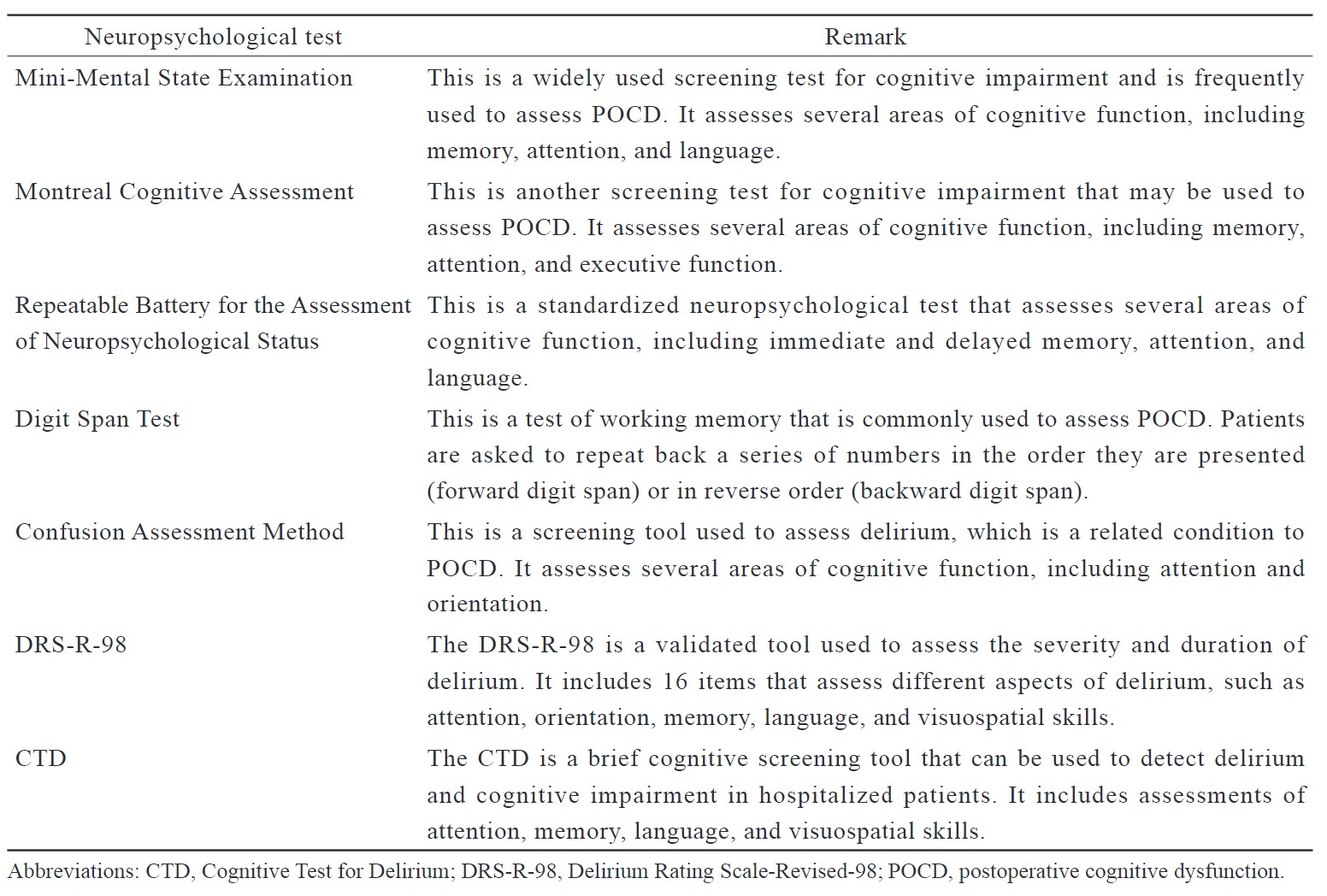
Download full-size image
Incidence and Risk Factors
POCD is a well-known complication after non-cardiac surgeries especially in the patients undergoing major surgeries and the geriatric age group. Incidence in non-cardiac surgery depends on the type of surgery, patient population, and other factors.
The etiology of POCD in non-cardiac surgery is not fully understood and is considered to be an interplay of multiple factors such as anesthesia, surgery-related inflammation and underlying health conditions. Some potential causes or contributing factors of POCD are mentioned. (Table 2)
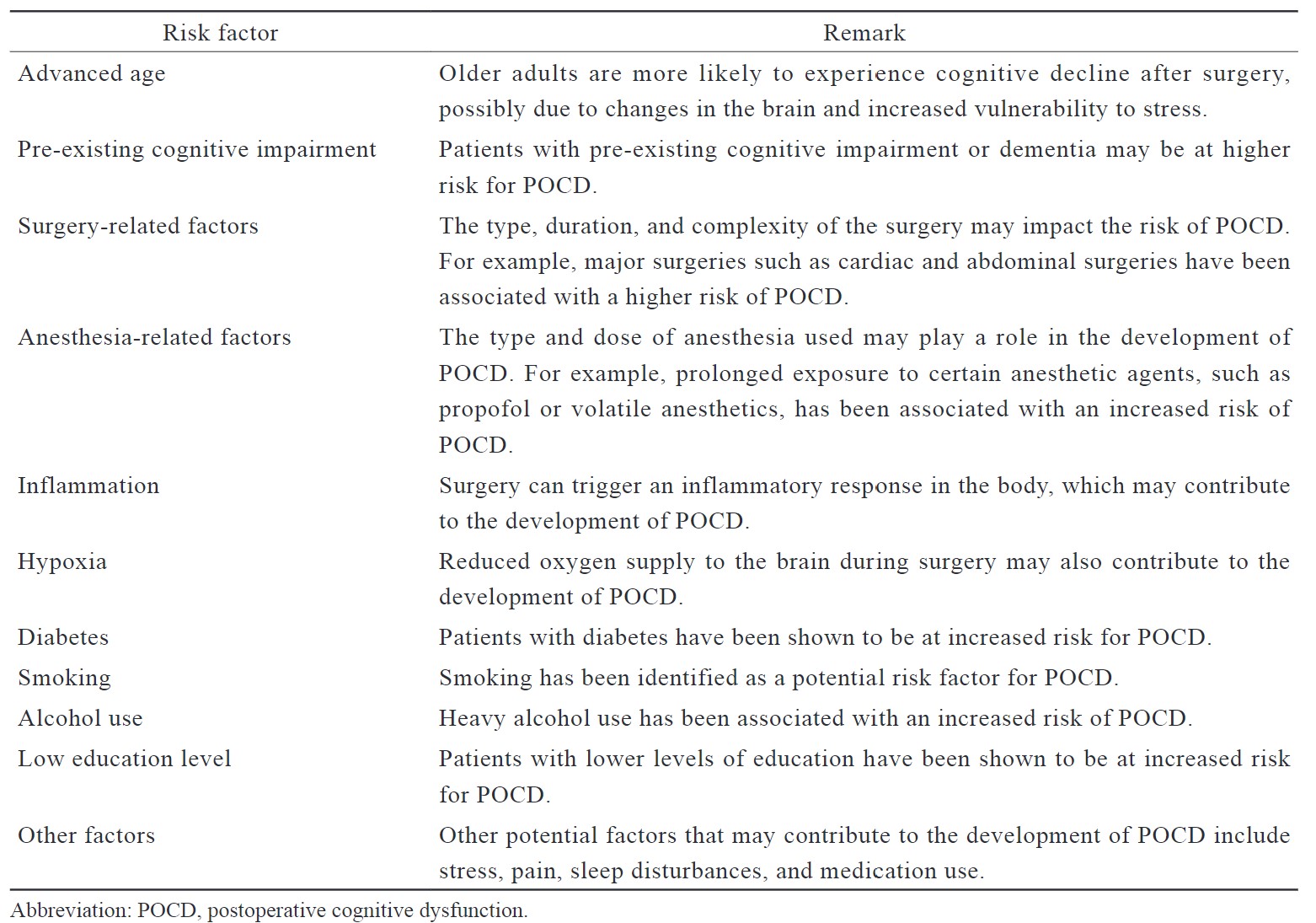
Download full-size image
POCD and postoperative delirium, and in particular hypoactive delirium, are often missed. There are large variations in incidence depending on multiple patient factors. Previous studies reported the incidence to range from 25% to 60% in clinical settings with geriatric age groups and even up to 73% post-cardiac surgery.11
Efforts to minimize the risk of POCD in non-cardiac surgery include careful monitoring of anesthesia and inflammation, as well as proactive management of underlying health conditions. Additionally, interventions such as cognitive rehabilitation, physical exercise, and social engagement may help to mitigate the effects of POCD in affected patients.
In non-cardiac surgery patients, a systematic review reported an incidence of 11.7% over a period of 3 months.12 Hip fractures with multiple underlying co-morbidities and age ≥ 65 with some degree of pre-existing cognitive impairment are at enhanced risk and incidence has been estimated to be 22%.9,13 Persistence of cognitive dysfunction for ≥ 6–12 months could be termed as long-term cognitive dysfunction. Long-term cognitive dysfunction as well as the risk of developing dementia is more in elderly patients receiving general anesthesia (GA).14-16 Previous studies have reported that delirium could be linked to long-term cognitive dysfunction or dementia.17,18
It has been noted in a number of previous studies that risk factors include elderly age, lower level of education, utilization of sedatives, previous history of depression & stroke, evidence of postoperative infection & pulmonary complications, lacunae on brain imaging and longer intraoperative duration of bispectral index (BIS) readings < 40.12,19,20
Krenk et al.21 observed that POCD can arise at any age, but manifestations are more severe and long-lasting in patients with age > 60 years. POCD is more prevalent in patients with coronary atherosclerosis or pre-existing dementia.22,23 It was observed in a study that incidence of POCD was highest in patients > 60 years and lowest in the age group 18–39 years. It was further noted that POCD was associated with increased mortality.24
It has been noted that a history of excessive alcohol intake was associated with an increased risk of POCD. 25 Genetic factors as well as low educational level are risk factors for the development of POCD.26
Pathophysiology
The exact pathophysiology of POCD is not well understood, and multiple mechanisms have been proposed. (Table 3)
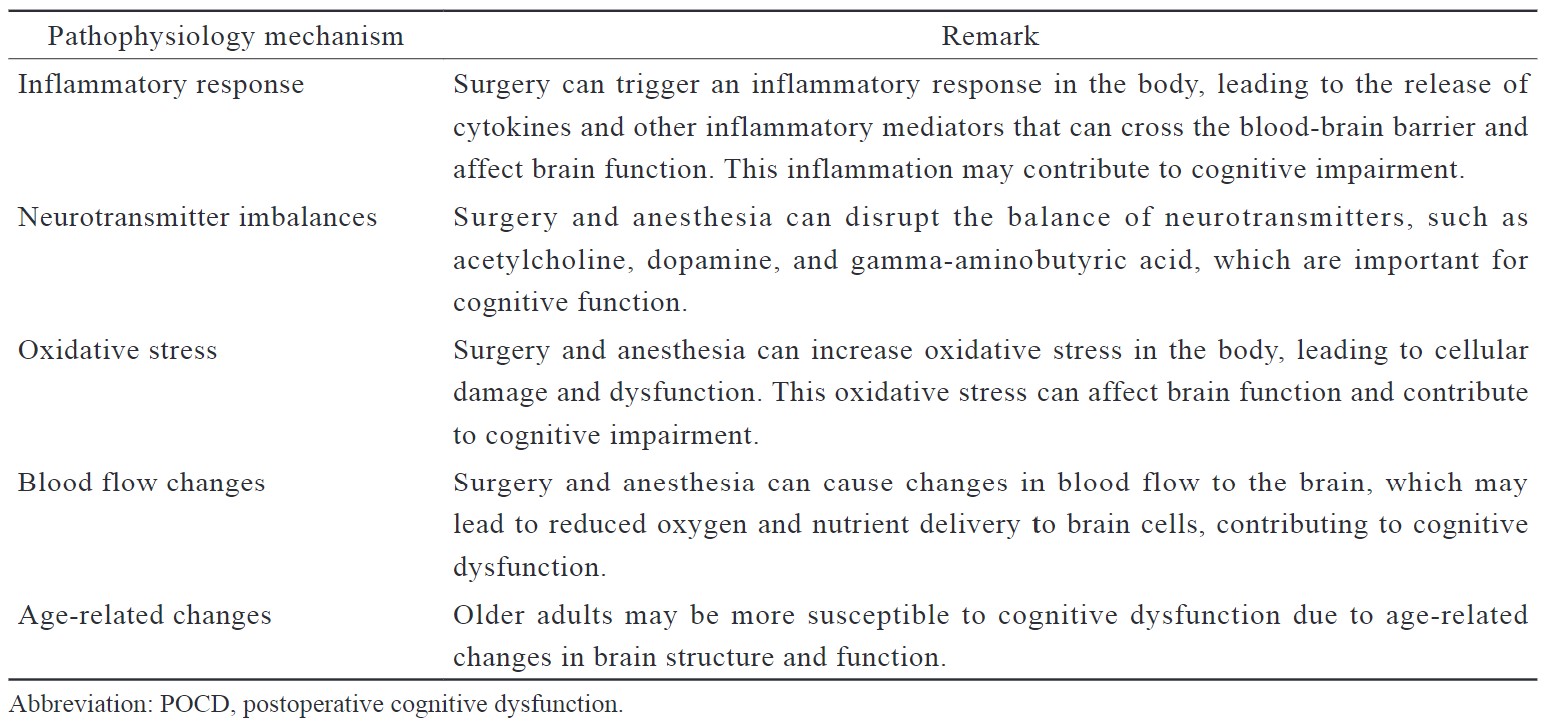
Download full-size image
Research has concentrated on the interaction between anesthetic agents and the pathology of Alzheimer’s disease. The pathological effects of tau proteins and extracellular amyloid plaques of Ab40 and Ab42 peptides result in increased neuronal death and loss of synapses, mostly of cholinergic neurones in the basal forebrain.27 These cholinergic neurons are vital in the formation and regulation of consciousness, learning and memory. Amyloid plaques in this region of the forebrain result in declining memory, reasoning, judgment and orientation.27
Animal models have demonstrated that isoflurane sevoflurane and desflurane promote neuronal death when associated with Alzheimer’s disease. One such study revealed that both normal mice and Transgenic Alzheimer’s disease mice when exposed to sevoflurane resulted in increased caspase-3 activation (apoptosis marker) in brain.28
Kline et al.23 studied whether surgery influences the course of dementia in Alzheimer’s disease. They found out in MRI scans 5–9 months post-surgery were associated with reduced volume of gray matter, atrophic changes in hippocampus, and increased size of ventricles. POCD was more pronounced in patients already having mild cognitive dysfunction prior to surgery.23
In cardiac surgeries, it has been reported that decreased cerebral oxygenation due to cerebral hypoperfusion or hypoxia is associated with POCD. Therefore, during cardiac surgery target mean arterial pressure is 80–90 mm Hg.29
Hypotension, hypoxia, and altered cerebral perfusion have been suggested as underlying mechanisms for POCD. The ISPOCD cohort study however didn’t find any association between either hypotension or hypoxia and POCD.
Terrando et al.30 suggested that surgery in mice leads to activation of TNF-
Biomarkers in POCD
• S100B Protein: It is associated with Alzheimer’s disease, autoimmune diseases, multiple sclerosis, schizophrenia, and cerebrovascular diseases.31 Higher concentrations are associated with the release of cytokines, apoptosis, neuroinflammation, and neurodegeneration.32 It has been reported that levels of S100B were significantly higher in patients who developed POCD undergoing total hip arthroplasty, transurethral resection of the prostate, and robot-assisted laparoscopic radical prostatectomy.33-35
• Interleukin-6 [IL-6]: In a normal scenario, low levels of IL-6 are present in the central nervous system. It has been found in animal models that a high concentration of IL-6 could inhibit neuronal synapses and hippocampal neurons in the dentate gyrus.36 Palta et al.37 reported that high plasma levels of IL-6 are associated with a higher incidence of cognitive dysfunction.
• Interleukin-1
• Interleukin-17 [IL-17]: IL-17 levels are increased in T cell-mediated inflammatory response and are closely related to the regulation of autoimmunity.40 It could induce the secretion of IL-1
• Tumor Necrosis Factor-
• Cyclooxygenase-2 and Prostaglandin E: Cyclooxygenases are heme-containing isoenzymes (COX-1 and COX-2) that catalyze the conversion of arachidonic acid to prostaglandin. COX-2 is present in the postsynaptic dendrites, cortical neurons, and spinal neurons. Induction of COX-2 induction is associated with neuroinflammation and neurodegeneration.50 COX-2 results in increased production of PGE2 as a result of synaptic activation, which causes glutamate release.51 The increase in glutamate causes a decrease in the number of small albumin-positive GABA cells and ultimately results in an imbalance between excitatory and inhibitory neurotransmission.52
Effects of Anesthesia
POCD is a common complication following surgery, particularly in older adults. It is characterized by a decline in cognitive function that occurs after surgery and can last for weeks or even months. The choice of anesthesia, inhalational anesthesia, or total intravenous anesthesia (TIVA) has been investigated as a potential risk factor for POCD.
The effect of inhalational agents on POCD has been extensively studied. Inhalational anesthetics, such as desflurane, sevoflurane, and isoflurane, are commonly used to induce and maintain anesthesia during surgery.
Some studies have suggested that inhalational anesthesia may be associated with a higher incidence of POCD compared to TIVA, while others have found no significant difference between the two types of anesthesia. Overall, the relationship between inhalational anesthesia and POCD is complex and not fully understood. Other factors, such as patient age, surgical procedure, and duration of anesthesia, may also play a role in the development of POCD. Further research is needed to fully understand the relationship between inhalational anesthesia and POCD, and to identify effective strategies for prevention and management of this complication.
The use of TIVA could be beneficial in POCD. It has been documented that propofol has positive effects on inhibiting inflammation by reducing levels of cytokines in plasma, in contrast to inhalational agents which promote inflammation in animal models.53,54
The results of these studies have been mixed, with some showing a higher incidence of POCD following inhalational anesthesia, while others have found no significant difference between the two types of anesthesia. Despite the mixed results, some experts suggest that TIVA may be a safer choice of anesthesia in patients at higher risk of POCD, such as elderly patients and those undergoing complex or prolonged surgery. However, further research is needed to fully understand the relationship between anesthesia type and POCD, and to identify effective strategies for prevention and management of this complication.
Many studies have been undertaken to compare the risk of POCD in GA vs. regional and neuraxial anesthesia techniques. A meta-analysis evaluated the incidence of POCD in GA vs. non-GA techniques which included spinal, epidural, regional, and the combination of GA plus neuraxial or regional anesthesia. It showed a non-significant increase of POCD incidence in GA as compared to non-GA techniques.55 Furthermore, an RCT conducted by Silbert et al.56 evaluated the incidence of POCD in patients undergoing extracorporeal shock wave lithotripsy. It was concluded that the type of anesthesia did not impact rates of POCD up to 3 months postoperatively. It was reported in the practice guidelines for perioperative brain health that based on current data, the evidence is insufficient to point out the benefit of regional anesthesia over GA regarding POCD.57 It has been observed that the use of TIVA can potentially decrease the incidence of POCD in patients undergoing thoracic surgery.58,59 Furthermore, TIVA has more neuroprotective effects when compared to sevoflurane in patients undergoing abdominal surgery and cancer surgery.60,61 However, many studies have reported that the use of propofol may be associated with worsened cognitive function sevoflurane.62-64 There are studies which haven’t demonstrated any differences in the incidence of POCD between propofol and sevoflurane.65,66
Depth of anethesia is an independent risk factor in the development of POCD. A meta-analysis compared reduced vs. increased depth of anesthesia monitored by BIS using either propofol or isoflurane for maintenance of anesthesia. It was found that there was no significant impact of depth of anesthesia on the development of POCD.67 The meta-analysis however included only 3 RCTs and didn’t likely represent the patient population at risk for POCD. Hou et al.68 studied the risk of POCD with varying depths of anesthesia in patients undergoing total knee replacement. Anesthesia in all patients was maintained using sevoflurane and propofol infusion. It was concluded that patients with BIS 40–50 had a higher incidence of POCD as compared to patients with BIS 55–65 in the immediate postoperative period. However, no significant difference was observed in postoperative days 3–7. BIS or entropy used for monitoring the depth of anesthesia don’t correlate with age or pre-existing cognitive dysfunction and therefore not reliable for assessing the risk of POCD in the patient population.69,70 Many studies identify a correlation between cerebral autoregulation and POCD, hypothermia and perioperative glycemic status as potential risk factors for POCD.71-73
The meta-analysis by Kim et al.74 used data from the Korean National Health Insurance Service. This was a large prospective cohort study which assessed the incidence of dementia in patients undergoing GA. It was concluded that GA was associated with the increased risk of developing dementia as compared to non-GA groups. It was also noted that the risk of dementia was higher with the use of desflurane and isoflurane, but lower with sevoflurane. The study design however would not be able to differentiate between the effects of anesthesia exposure from that of surgical stress and other factors such as perioperative hypothermia, use of narcotics and other medications.
The 5th International Perioperative Neurotoxicity Working groups recommendations are as follows57:
• Individuals over the age of 65 should be informed of the risk of postoperative delirium and perioperative neurocognitive disorder prior to their procedure.
• Baseline cognitive function should be assessed preoperatively using a brief screening tool.
• Current literature is insufficient to define a specific anesthetic regimen to decrease the risk of perioperative neurocognitive disorder; however, cautious use or avoidance of medications such as first-generation antihistamines, centrally acting anticholinergics, benzodiazepines, and meperidine was recommended. Avoid relative hypotension, maintenance of normothermia, use age-adjusted minimal alveolar concentration of volatile anesthetic agents, and use EEG-based depth of anesthesia monitoring to titrate anesthetic delivery.
• Additional research is required to determine the feasibility, efficacy, and cost- effectiveness of postoperative follow-up to assess cognitive outcomes.
Role of Dexmedetomidine
The exact mechanism of action of dexmedetomidine regarding POCD isn’t clearly understood, but it can be attributed to a reduction in dose requirements of opioids and anesthetic drugs, systemic stress-response modulation, promotion of natural sleep cycle, and direct neuroprotection.75,76 Su et al.76 have reported that low-dose dexmedetomidine in the postoperative period is associated with a decrease in the incidence of delirium among the geriatric population. A meta-analysis found that dexmedetomidine may result in a decreased incidence of cognitive dysfunction and improvement in mini-mental state exam (MMSE) scores.77 Deiner et al.78 didn’t observe any significant difference in the incidence of postoperative delirium and POCD in patients receiving dexmedetomidine infusion during intraoperative and postoperative periods. Yu et al.79 in a meta-analysis concluded that dexmedetomidine reduces the risk of POCD in older patients undergoing major surgeries under GA.
Limitations
Studying the relationship between POCD and anesthesia presents several challenges and limitations. Human researches must adhere to strict ethical guidelines, which can sometimes limit the scope and methodology of the studies. Gathering a large enough sample size can be difficult due to the specialized nature of the population. However, a small sample size can limit the statistical power of the study. Anesthesia is often administered alongside various other medications and numerous interventions are being done during surgery. It, therefore, becomes challenging to isolate the specific effects of anesthesia on cognitive function. Also, there are a variety of anesthetic agents, with different characteristics and potential effects on cognition. Hence, studying the correlation between POCD and anesthesia further becomes complicated.
Understanding the long-term effects of anesthesia on cognitive function requires longitudinal studies that track patients over extended periods. However, such studies can be resource-intensive and may face challenges with participant retention. Assessing cognitive function accurately and consistently across different individuals and settings is a difficult task. The choice of assessment tools and methods can impact the results and comparability of studies. There may exist a publication bias, as studies that find significant correlations between anesthesia and POCD may be more likely to be published than those with null results.
Various confounding factors that are correlated with both anesthesia and POCD can lead to misleading conclusions about the relationship between them. Several confounding factors that need to be considered are age, pre-existing cognitive dysfunction, comorbidities, type and duration of surgery, choice of anesthetic agent and technique, trauma caused due to surgery, and other medications given alongside anesthetic agents.
The type and duration of surgery can affect the stress response, inflammation, and overall physiological impact on the body, which may influence cognitive function independent of anesthesia. Postoperative complications such as infection, hypoxia, and hypotension can also affect postoperative cognitive function. Psychological factors such as anxiety, depression, and stress can impact cognitive function in the perioperative period. Environmental factors such as noise, lighting, and quality of care in the perioperative environment can affect cognitive function.
Addressing these limitations requires careful study design, rigorous methodology, collaboration across disciplines, and ongoing research efforts to better understand the complex relationship between anesthesia and POCD.
Conclusion
In summary, POCD is a recognized complication of non-cardiac surgery that can have significant impacts on patients’ cognitive function and quality of life after surgery. Efforts to minimize the risk of POCD and support affected patients are important considerations in perioperative care for all types of surgeries. It remains doubtful whether anesthesia itself or surgical or patient factors might be responsible for POCD. There is conflict among studies regarding anesthesia exposure and the risk of developing POCD. The studies which assessed the risk profile of various anesthetic techniques are heterogeneous, and therefore no strong evidence is present in favor of any technique.
References
| 1 |
Bedford PD.
Adverse cerebral effects of anaesthesia on old people.
Lancet. 1955;269(6884):259-263.
|
| 2 |
National Institute for Health and Care Excellence.
Delirium: prevention, diagnosis and management in hospital and long-term care.
|
| 3 |
Fong TG, Tulebaev SR, Inouye SK.
Delirium in elderly adults: diagnosis, prevention and treatment.
Nat Rev Neurol. 2009;5(4):210-220.
|
| 4 |
American Psychiatric Association.
Neurocognitive Disorders.
|
| 5 |
Leung JM.
Postoperative delirium: are there modifiable risk factors?
Eur J Anaesthesiol. 2010;27(5):403-405.
|
| 6 |
Ansaloni L, Catena F, Chattat R, et al.
Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery.
Br J Surg. 2010;97(2):273-280.
|
| 7 |
Hove LD, Steinmetz J.
Inadequate recovery: when emergence from anaesthesia is not really smooth.
Minerva Anestesiol. 2010;76(6):385-386.
|
| 8 |
Radtke FM, Franck M, Hagemann L, Seeling M, Wernecke KD, Spies CD.
Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence.
Minerva Anestesiol. 2010;76(6):394-403.
|
| 9 |
Jansen CL, Absalom AR, de Bock GH, van Leeuwen BL, Izaks GJ.
Performance and agreement of risk stratification instruments for postoperative delirium in persons aged 50 years or older.
PLoS One. 2014;9(12):e113946.
|
| 10 |
Sauër AC, Veldhuijzen DS, Ottens TH, Slooter AJC, Kalkman CJ, van Dijk D.
Association between delirium and cognitive change after cardiac surgery.
Br J Anaesth. 2017;119(2):308-315.
|
| 11 |
Rudolph JL, Inouye SK, Jones RN, et al.
Delirium: an independent predictor of functional decline after cardiac surgery.
J Am Geriatr Soc. 2010;58(4):643-649.
|
| 12 |
Paredes S, Cortínez L, Contreras V, Silbert B.
Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review.
Acta Anaesthesiol Scand. 2016;60(8):1043-1058.
|
| 13 |
Chow WB, Rosenthal RA, Merkow RP, et al.
Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society.
J Am Coll Surg. 2012;215(4):453-466.
|
| 14 |
Tsai TL, Sands LP, Leung JM.
An update on postoperative cognitive dysfunction.
Adv Anesth. 2010;28(1):269-284.
|
| 15 |
Sztark F, Le Goff M, André D, Ritchie K, Dartigues JF, Helmer C.
Exposure to general anaesthesia could increase the risk of dementia in elderly: 18AP1-4.
Eur J Anaesthesiol. 2013;30:245.
|
| 16 |
Sprung J, Jankowski CJ, Roberts RO, et al.
Anesthesia and incident dementia: a population-based, nested, case-control study.
Mayo Clin Proc. 2013;88(6):552-561.
|
| 17 |
Aranake-Chrisinger A, Avidan MS.
Postoperative delirium portends descent to dementia.
Br J Anaesth. 2017;119(2):285-288.
|
| 18 |
Sprung J, Roberts RO, Weingarten TN, et al.
Postoperative delirium in elderly patients is associated with subsequent cognitive impairment.
Br J Anaesth. 2017;119(2):316-323.
|
| 19 |
Brown C IV, Deiner S.
Perioperative cognitive protection.
Br J Anaesth. 2016;117(Suppl 3):iii52-iii61.
|
| 20 |
Lloyd DG, Ma D, Vizcaychipi MP.
Cognitive decline after anaesthesia and critical care.
Continuing Education in Anaesthesia Critical Care & Pain. 2012;12(3):105-109.
|
| 21 |
Krenk L, Rasmussen LS, Kehlet H.
New insights into the pathophysiology of postoperative cognitive dysfunction.
Acta Anaesthesiol Scand. 2010;54(8):951-956.
|
| 22 |
Selnes OA, Grega MA, Bailey MM, et al.
Cognition 6 years after surgical or medical therapy for coronary artery disease.
Ann Neurol. 2008;63(5):581-590.
|
| 23 |
Kline RP, Pirraglia E, Cheng H, et al.
Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment.
Anesthesiology. 2012;116(3):603-612.
|
| 24 |
Monk TG, Weldon BC, Garvan CW, et al.
Predictors of cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 2008;108(1):18-30.
|
| 25 |
Hudetz JA, Iqbal Z, Gandhi SD, et al.
Postoperative cognitive dysfunction in older patients with a history of alcohol abuse.
Anesthesiology. 2007;106(3):423-430.
|
| 26 |
Ghoneim MM, Block RI.
Clinical, methodological and theoretical issues in the assessment of cognition after anaesthesia and surgery: a review.
Eur J Anaesthesiol. 2012;29(9):409-422.
|
| 27 |
Jiang J, Jiang H.
Effect of the inhaled anesthetics isoflurane, sevoflurane and desflurane on the neuropathogenesis of Alzheimer’s disease (review).
Mol Med Rep. 2015;12(1):3-12.
|
| 28 |
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z.
Anesthetic sevoflurane causes neurotoxicity differently in neonatal naïve and Alzheimer disease transgenic mice.
Anesthesiology. 2010;112(6):1404-1416.
|
| 29 |
van Harten AE, Scheeren TWL, Absalom AR.
A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia.
Anaesthesia. 2012;67(3):280-293.
|
| 30 |
Terrando N, Eriksson LI, Ryu JK, et al.
Resolving postoperative neuroinflammation and cognitive decline.
Ann Neurol. 2011;70(6):986-995.
|
| 31 |
Zhang XY, Xiu MH, Song C, et al.
Increased serum S100B in never-medicated and medicated schizophrenic patients.
J Psychiatr Res. 2010;44(16):1236-1240.
|
| 32 |
Kabadi SV, Stoica BA, Zimmer DB, et al.
S100B inhibition reduces behavioral and pathologic changes in experimental traumatic brain injury.
J Cereb Blood Flow Metab. 2015;35(12):2010-2020.
|
| 33 |
Gan J, Tu Q, Miao S, et al.
Effects of oxycodone applied for patient-controlled analgesia on postoperative cognitive function in elderly patients undergoing total hip arthroplasty: a randomized controlled clinical trial.
Aging Clin Exp Res. 2020;32(2):329-337.
|
| 34 |
Chi YL, Li ZS, Lin CS, Wang Q, Zhou YK.
Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia.
Eur Rev Med Pharmacol Sci. 2017;21(6):1346-1354.
|
| 35 |
Kavrut Ozturk N, Kavakli AS, Arslan U, Aykal G, Savas M.
S100B level and cognitive dysfunction after robotic-assisted laparoscopic radical prostatectomy procedures: a prospective observational study.
Braz J Anesthesiol. 2020;70(6):573-582.
|
| 36 |
Balschun D, Wetzel W, Del Rey A, et al.
Interleukin-6: a cytokine to forget.
FASEB J. 2004;18(14):1788-1790.
|
| 37 |
Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC.
Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the Women’s Health and Aging Study II.
J Gerontol A Biol Sci Med Sci. 2015;70(7):873-878.
|
| 38 |
Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF.
Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats.
J Neurosci. 2012;32(42):14641-14648.
|
| 39 |
Skelly DT, Griffin ÉW, Murray CL, et al.
Correction: Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms.
Mol Psychiatry. 2019;24(10):1566.
|
| 40 |
Iwakura Y, Ishigame H, Saijo S, Nakae S.
Functional specialization of interleukin-17 family members.
Immunity. 2011;34(2):149-162.
|
| 41 |
Ruiz de Morales JMG, Puig L, Daudén E, et al.
Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies.
Autoimmun Rev. 2020;19(1):102429.
|
| 42 |
Dubenko OE, Chyniak OS, Potapov OO.
Levels of proinflammatory cytokines IL-17 and IL-23 in patients with Alzheimer’s disease, mild cognitive impairment and vascular dementia.
Wiad Lek. 2021;74(1):68-71.
|
| 43 |
Bălaşa R, Bajko Z, Huţanu A.
Serum levels of IL-17A in patients with relapsing-remitting multiple sclerosis treated with interferon-β.
Mult Scler. 2013;19(7):885-890.
|
| 44 |
Zhang J, Mao X, Zhou T, Cheng X, Lin Y.
IL-17A contributes to brain ischemia reperfusion injury through calpain-TRPC6 pathway in mice.
Neuroscience. 2014;274:419-428.
|
| 45 |
Tian A, Ma H, Zhang R, et al.
Interleukin17A promotes postoperative cognitive dysfunction by triggering β-Amyloid accumulation via the transforming growth factor-β (TGFβ)/smad signaling pathway.
PLoS One. 2015;10(10):e0141596.
|
| 46 |
Pribiag H, Stellwagen D.
TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors.
J Neurosci. 2013;33(40):15879-15893.
|
| 47 |
Yang C, Zhu B, Ding J, Wang ZG.
Isoflurane anesthesia aggravates cognitive impairment in streptozotocin-induced diabetic rats.
Int J Clin Exp Med. 2014;7(4):903-910.
|
| 48 |
Jiang J, Lv X, Liang B, Jiang H.
Circulating TNF-α levels increased and correlated negatively with IGF-I in postoperative cognitive dysfunction.
Neurol Sci. 2017;38(8):1391-1392.
|
| 49 |
Sun S, Sun D, Yang L, Han J, Liu R, Wang L.
Dose-dependent effects of intravenous methoxamine infusion during hip-joint replacement surgery on postoperative cognitive dysfunction and blood TNF-α level in elderly patients: a randomized controlled trial.
BMC Anesthesiol. 2017;17(1):75.
|
| 50 |
Font-Nieves M, Sans-Fons MG, Gorina R, et al.
Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes.
J Biol Chem. 2012;287(9):6454-6468.
|
| 51 |
Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C.
Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor.
J Neurosci. 2005;25(43):9858-9870.
|
| 52 |
Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA.
MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase.
J Neuroimmune Pharmacol. 2013;8(1):58-65.
|
| 53 |
Lee CJ, Tai YT, Lin YL, Chen RM.
Molecular mechanisms of propofol-involved suppression of no biosynthesis and inducible iNOS gene expression in LPS-stimulated macrophage-like raw 264.
Shock. 2010;33(1):93-100.
|
| 54 |
Skvarc DR, Berk M, Byrne LK, et al.
Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies.
Neurosci Biobehav Rev. 2018;84:116-133.
|
| 55 |
Mason SE, Noel-Storr A, Ritchie CW.
The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis.
J Alzheimers Dis. 2010;22(Suppl 3):67-79.
|
| 56 |
Silbert BS, Evered LA, Scott DA.
Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy.
Br J Anaesth. 2014;113(5):784-791.
|
| 57 |
Berger M, Schenning KJ, Brown CH, et al.
Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group.
Anesth Analg. 2018;127(6):1406-1413.
|
| 58 |
Yu W.
Anesthesia with propofol and sevoflurane on postoperative cognitive function of elderly patients undergoing general thoracic surgery.
Pak J Pharm Sci. 2017;30(3 Special):1107-1110.
|
| 59 |
Tian HT, Duan XH, Yang YF, Wang Y, Bai QL, Zhang X.
Effects of propofol or sevoflurane anesthesia on the perioperative inflammatory response, pulmonary function and cognitive function in patients receiving lung cancer resection.
Eur Rev Med Pharmacol Sci. 2017;21(23):5515-5522.
|
| 60 |
Ding F, Wang X, Zhang L, Li J, Liu F, Wang L.
Effect of propofol-based total intravenous anaesthesia on postoperative cognitive function and sleep quality in elderly patients.
Int J Clin Pract. 2021;75(7):e14266.
|
| 61 |
Zhang Y, Shan GJ, Zhang YX, et al.
Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults.
Br J Anaesth. 2018;121(3):595-604.
|
| 62 |
Zhiguo Y, Nanxiang Z, Jinyu M.
Analysis of comparative anesthetic effects of sevoflurane and propofol on lung and cognitive functions.
Pak J Pharm Sci. 2019;32(5 Special):2423-2426.
|
| 63 |
Qin Y, Ni J, Kang L, Zhong Z, Wang L, Yin S.
Sevoflurane effect on cognitive function and the expression of oxidative stress response proteins in elderly patients undergoing radical surgery for lung cancer.
J Coll Physicians Surg Pak. 2019;29(1):12-15.
|
| 64 |
Sun H, Zhang G, Ai B, et al.
A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer.
BMC Cancer. 2019;19(1):1248.
|
| 65 |
Egawa J, Inoue S, Nishiwada T, et al.
Effects of anesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial.
Can J Anaesth. 2016;63(10):1161-1169.
|
| 66 |
Li Y, Chen D, Wang H, et al.
Intravenous versus volatile anesthetic effects on postoperative cognition in elderly patients undergoing laparoscopic abdominal surgery.
Anesthesiology. 2021;134(3):381-394.
|
| 67 |
Lu X, Jin X, Yang S, Xia Y.
The correlation of the depth of anesthesia and postoperative cognitive impairment: a meta-analysis based on randomized controlled trials.
J Clin Anesth. 2018;45:55-59.
|
| 68 |
Hou R, Wang H, Chen L, Qiu Y, Li S.
POCD in patients receiving total knee replacement under deep vs light anesthesia: a randomized controlled trial.
Brain Behav. 2018;8(2):e00910.
|
| 69 |
Erdogan MA, Demirbilek S, Erdil F, et al.
The effects of cognitive impairment on anaesthetic requirement in the elderly.
Eur J Anaesthesiol. 2012;29(7):326-331.
|
| 70 |
Purdon PL, Pavone KJ, Akeju O, et al.
The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia.
Br J Anaesth. 2015;115(Suppl 1):i46-i57.
|
| 71 |
Goettel N, Burkhart CS, Rossi A, et al.
Associations between impaired cerebral blood flow autoregulation, cerebral oxygenation, and biomarkers of brain injury and postoperative cognitive dysfunction in elderly patients after major noncardiac surgery.
Anesth Analg. 2017;124(3):934-942.
|
| 72 |
Gong GL, Liu B, Wu JX, Li JY, Shu BQ, You ZJ.
Postoperative cognitive dysfunction induced by different surgical methods and its risk factors.
Am Surg. 2018;84(9):1531-1537.
|
| 73 |
Shoair OA, Grasso Ii MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW.
Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study.
J Anaesthesiol Clin Pharmacol. 2015;31(1):30-36.
|
| 74 |
Kim CT, Myung W, Lewis M, et al.
Exposure to general anesthesia and risk of dementia: a nationwide population-based cohort study.
J Alzheimers Dis. 2018;63(1):395-405.
|
| 75 |
Mo Y, Zimmermann AE.
Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients.
Ann Pharmacother. 2013;47(6):869-876.
|
| 76 |
Su X, Meng ZT, Wu XH, et al.
Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial.
Lancet. 2016;388(10054):1893-1902.
|
| 77 |
Zhou C, Zhu Y, Liu Z, Ruan L.
Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: a meta-analysis.
J Int Med Res. 2016;44(6):1182-1190.
|
| 78 |
Deiner S, Luo X, Lin HM, et al.
Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial.
JAMA Surg. 2017;152(8):e171505.
|
| 79 |
Yu H, Kang H, Fan J, Cao G, Liu B.
Influence of dexmedetomidine on postoperative cognitive dysfunction in the elderly: a meta-analysis of randomized controlled trials.
Brain Behav. 2022;12(8):e2665.
|