Abstract
Many cancer patients have their main tumors surgically removed with the goal of curing them, or as part of palliative care. When a patient receives general anesthesia, the side effects of the administered chemotherapy regimens must be considered, especially those that may have significant repercussions during anesthetic care. The aim of this study is to review the existing literature to identify adverse effects of chemotherapy and known interactions between chemotherapy and anesthetic drugs, analyze the mechanisms underlying these interactions, and discuss relevant clinical implications. This integrative review searched for articles using the search terms: chemotherapy, anesthetic drugs, interaction, adverse effects, and drug combination. Negative perioperative outcomes may be related to the chemotherapy. Regarding the type of oncological surgery, factors such as blood loss, hypothermia, and extended operative time influence the increase in morbimortality. Many anesthetic drugs are related to immunomodulation and interact with chemotherapy, which may get better or worse cancer patients. It is crucial for anesthetists to have a comprehensive understanding of the potential adverse effects of chemotherapy drugs and their interactions with anesthetics.
Keywords
adjuvant chemotherapy, adverse drug event, anesthetics agents, antineoplastic drugs, cancer treatment protocol
Introduction
Many cancer patients have their main tumors surgically removed with the goal of curing them, or they may require surgery as part of palliative care for an advanced stage cancer.1 Chemotherapy can be used as neoadjuvant therapy before surgical excision to ease the burden of the tumor, kill micrometastases, and possibly reduce the viability of tumor cells released during surgical manipulation; adjuvant therapy after surgical excision to try to eliminate clinically undetectable residual tumor burden; or palliative therapy given at any time to relieve patient symptoms.2,3
As an alternative, cancer patients may need emergency operations frequently because of side effects from their continuous chemotherapy or surgeries unrelated to their tumor, as well as difficulties from their condition as it progresses. Therefore, when such a patient obtains general anesthesia, the numerous side effects of the provided chemotherapy regimens need to be taken into account, especially those that may have major repercussions during the anesthetic care.1
Conventional cancer drugs have a very narrow therapeutic index. They are highly toxic to both the targeted cancer cells and non-targeted cells.2 Intestinal mucosa and bone marrow, for instance, have rapid cellular turnover and, for that, are also adversely affected by drugs that interfere with DNA replication. Microtubule function will be impacted by substances that alter microtubule activity both during cellular division and during other microtubule activities, such as the assembly and distribution of synaptic vesicles at the neuromuscular junction. Peripheral neuropathies are caused by disruption of synaptic vesicles. Last but not least, substances that cause cardiomyopathies also harm cells with high metabolic activity, such as cardiac myocytes, by producing oxygen free radicals that destroy quickly dividing cells.2
Three main categories can be used to classify medications that are used to kill cancer cells. Alkylating agents, antimetabolites, antineoplastic antibiotics, and topoisomerase inhibitors are within the first category of chemicals that directly harm DNA and/or ribonucleic acid (RNA). The second group prevents dividing cells from proliferating by disrupting microtubule activity. Targeted therapies, also known as precision medicine therapies, growth inhibitors, and molecular therapies, are included in the third group. Targeted therapies block particular biological enzymes that are specific to or overexpressed in cancerous cells. Targeted therapies can also be chemicals designed to bind to particular cell membrane antigens in order to mark the cell and make it easier for the immune system to identify the tumor. Table 1 shows the three main categories of chemotherapeutic drugs, based on their mechanism of action.2
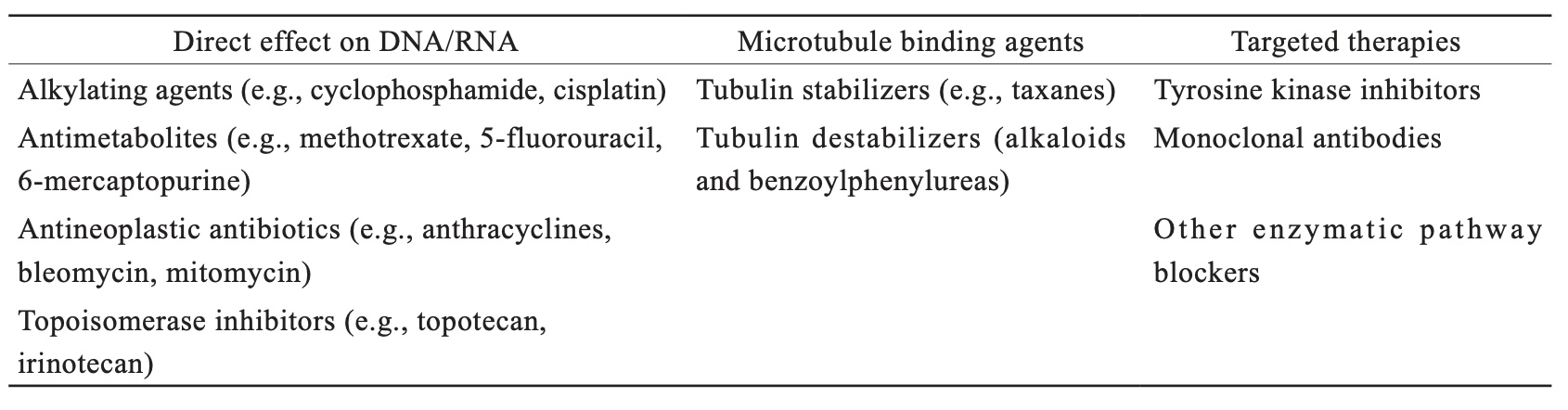
Download full-size image
The aim of this study is to review the existing literature to identify adverse effects of chemotherapy and known interactions between chemotherapy and anesthetic drugs, analyze the mechanisms underlying these interactions, and discuss relevant clinical implications.
Methodology
A comprehensive literature search was conducted in the PubMed and Scopus databases using the search terms: chemotherapy, anesthetic drugs, interaction, adverse effects, and drug combination. The search included studies published from 1978 and 2023. Original studies, systematic reviews, and relevant clinical guidelines were included. Studies in English, French, and Spanish were included. Narrative review papers were included if they described the main adverse effects of the antineoplastic drugs and the consequences for the patient submitted to a surgical procedure or the interactions between the antineoplastic drugs and the anesthetics and analgesic drugs. A variety of studies, including quantitative, qualitative, and mixed-method approaches, were considered to explore the interactions between antineoplastic drugs and anesthetics and how these interactions could impact the intraoperative period and prognosis of cancer patients. Papers were excluded if they did not fit into the conceptual framework of the study. Studies with animals were also excluded.
Studies considered relevant were evaluated in full. The following data were extracted from the included studies: authors, year of publication, studied population, type of chemotherapy, type of anesthetic drug, main results on the interaction, proposed mechanisms, and clinical recommendations. Relevant information was grouped and presented in a narrative form. Figure 1 is a flow diagram with the studies’ selection, inclusion, and exclusion4.
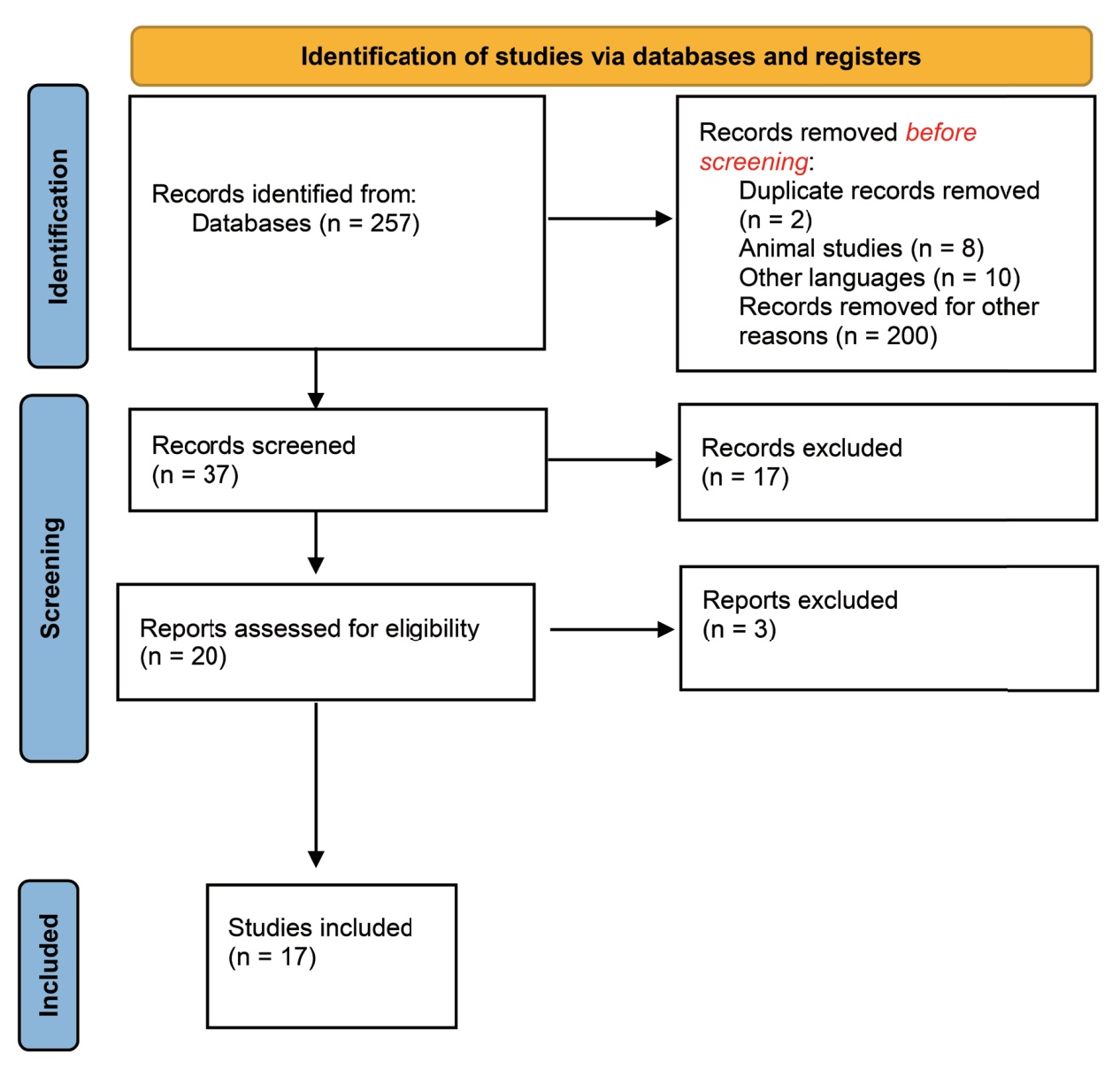
Download full-size image
Discussion
It is imperative to do a thorough pre-operative examination of patients who have used chemotherapy in the past because anti-cancer chemotherapeutic medications have a history of causing generalized and specific organ toxicities as well as a variety of unanticipated or life-threatening peri-operative complications.5
Myocyte death, caused by a combination of factors, can be detected through histopathological analysis, biochemical markers, imaging tests, or clinical signs of malfunction. The primary causes of cardiotoxicity include the loss of myofibrils, vacuolization of myocytes, and altered topoisomerase activity. Silent myocardial ischemia may result from coronary vasospasm, endothelial toxicity-related thrombosis, or takotsubo cardiomyopathy, and can lead to arrhythmias due to myocardial ischemia, direct heart tissue damage, or chemotherapy-induced electrolyte imbalances. Taxanes like paclitaxel and docetaxel are associated with arrhythmias, atrioventricular block, left bundle branch block, bradycardia, and in severe cases, ventricular tachycardia. Tyrosine kinase inhibitors can damage cardiac myocytes, leading to apoptosis, cardiomyopathy, congestive heart failure, and QT prolongation, increasing the risk of Torsades de pointes. Anthracyclines can cause early, reversible toxicity leading to a temporary decrease in ejection fraction, arrhythmias, hypotension, ischemia, myocarditis, pericarditis, and contractile failure.1 Research has indicated that patients with normal resting cardiac function may still experience minor changes in cardiac function after chemotherapy with anthracyclines, which become noticeable only after anesthesia or exercise.6
According to Huettemann et al.7, children who have previously received anthracycline with normal myocardial function at rest may exhibit a more pronounced cardiodepressive effect of anesthetics as evidence of subclinical cardiotoxicity.
Chemotherapy for antineoplastic diseases usually causes toxicity in the lungs. Approximately 20% of people receiving chemotherapy experience pulmonary side effects. Numerous pathophysiologic mechanisms have been put forth to explain antineoplastic agent-induced lung injury, including endothelial dysfunction with subsequent capillary leak syndrome, recruitment of inflammatory cells because of direct injury to pneumocytes, free oxygen radicals causing direct lung tissue injury, and impairing lung repair. The pathogenesis of antineoplastic agent-induced lung injury is unknown. Patients often have a nonproductive cough, dyspnea, fever, or weight loss when they first show clinically. Restrictive lung physiology is frequently seen in pulmonary function tests, which show lower lung volumes and diffusion capacity.8,9
Up to 25% of patients may develop lung fibrosis or interstitial pneumonia as a result of certain chemotherapy drugs. Bleomycin (first category) is the chemotherapeutic drug that has been shown to cause lung damage most thoroughly. The symptoms of bleomycin toxicity include pulmonary fibrosis that progresses and causes severe restrictive lung disease.8,10
Iron and bleomycin combine to create oxygen-free radicals. The bleomycin hydrolase enzyme, which is present in every part of the body but the skin and lungs, inactivates it. Alveolar epithelial cells in the lungs, where aminohydrolase activity is particularly low, collect bleomycin. Bleomycin damages the pulmonary endothelium, increasing capillary permeability and generating edema, and the edema causes even more bleomycin to accumulate.8 Type I alveolar epithelial necrosis promotes enhanced macrophage migration, which in turn attracts more inflammatory
cells that discharge oxygen radicals and activate fibroblast activity. Up to 25% of patients who have previously received bleomycin therapy run the risk of developing post-operative respiratory failure, needing prolonged post-operative tracheal intubation. Excessive fluid intake, perioperative transfusion, a decline in vital capacity, and the length of the surgery are indicators of post-operative respiratory insufficiency.5
Acute respiratory distress syndrome and potentiation of bleomycin pneumotoxicity have both been linked to anesthetic exposure to high oxygen concentrations. In conclusion, the anesthesiologist must evaluate if the oncology patient has symptomatic pulmonary dysfunction and take into account the likelihood of occult respiratory compromise.8
Acute kidney injury (AKI) from chemotherapy might result from the kidneys’ frequent elimination of chemotherapeutic drugs. Some agents such as paclitaxel, cisplatin, cyclophosphamide, methotrexate, and vincristine are associated with AKI that can affect the proximal tubules, whose symptoms include phosphate wasting, Fanconi syndrome (hypophosphataemia, hypokalaemia, glycosuria, and proteinuria) and magnesium wasting. In one-third of cases, the usage of cisplatin (first category) is linked to nephrotoxicity.1
Chemotherapeutic drugs may influence the immune system in a variety of ways, from stimulatory to immunosuppressive. Both the innate and adaptive immune systems are impacted. Myelosuppression, which causes chemotherapy-induced neutropenia, may have an impact on the innate immune system.11 The chances of sepsis and systemic infections rise with severe neutropenia. The condition known as pancytopenia, which is marked by a decrease in erythrocytes, leucocytes, and platelets, may have substantial effects on perioperative treatment. The risk of bleeding and opportunistic infection increases during the perioperative phase, while oxygen-carrying capacity decreases. Inducing an inflammatory, procoagulant, antifibrinolytic, and pro-aggregative reaction that results in venous thrombosis is a common side effect of both cancer and neoadjuvant therapy. Anticoagulant proteins including antithrombin and protein C are less concentrated as a result of the inflammatory response. The expression of tissue factors increases, activating the procoagulant reaction.11
Numerous pertinent adverse responses, such as endocrine, cardiac, and pulmonary toxicities, may be brought on by various immunotherapies during the perioperative period. Immune checkpoint inhibitors (third group) have the potential to cause endocrinopathies, the most prevalent of which is hypophysitis, an inflammation of the pituitary gland. Numerous clinical manifestations, such as hypothyroidism, adrenal insufficiency, hypogonadism, and diabetes insipidus, can be brought on by this illness.11
Most monoclonal antibodies (alemtuzumab, cetuximab, and rituximab) can cause a large amount of cytokine release after rapid infusion, which can lead to an allergic reaction that can result in hypotension, anaphylactic shock, and in 3%–9% of cases, mortality.1 Metotrexate is related to hypersensitivity reactions and eosinophilia.10
Table 2 shows the chemotherapeutic classes and their main side effects of interest to anesthetists during surgery.
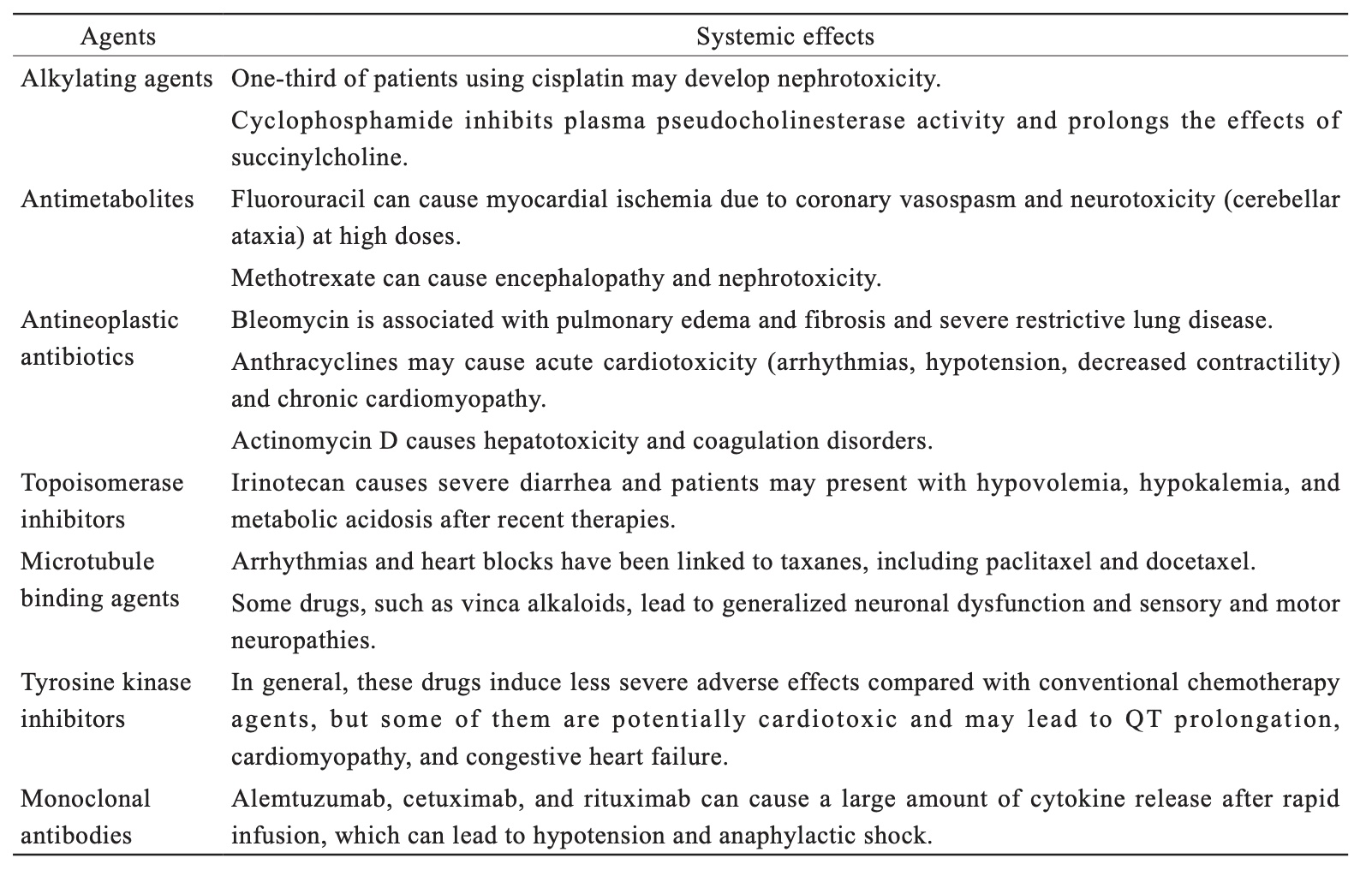
Download full-size image
When treating cancer, the anesthesiologist faces some challenges, including physiological disruptions, tumor-related symptoms, and toxicity in conventional chemotherapy.
Various anesthetics and chemotherapy drugs interact differently depending on the type of cancer, according to studies.12
The liver plays a major role in the biotransformation of various drugs such as analgesics, sedatives, and antitumor drugs. Therefore, patients receiving certain types of chemotherapy would be sensitive to normally safe doses of anesthetic agents.5
Drug interaction occurs due to several mechanisms. The presence of one drug may alter the uptake or absorption of another, a competition between two drugs for the same binding site on plasma proteins may occur, two drugs may compete for the same receptors and the administration of one drug may alter the metabolism of another, with possible changes in the action of both. Moreover, the administration of a drug may alter the excretion of another.13
Anesthetists should be aware of potential motor and sensory neuropathies in patients who have taken microtubule inhibitors and the role they may play in the use and supervision of depolarizing and nondepolarizing muscle relaxants.12 Cyclophosphamide has the ability to prolong the effects of succinylcholine while specifically inhibiting plasma pseudocholinesterase activity.12
Nonsteroidal anti-inflammatory medications (NSAIDs) and methotrexate can interact negatively. Although the exact mechanism of this interaction is unclear, NSAIDs are known to reduce methotrexate excretion. Although the mechanism is yet unknown, it has been hypothesized that there is competition for receptor sites at renal tubular excretion. As a result, patients may experience potentially deadly side effects.5,14
Cisplatin is one of the most recognizable agents associated with nephrotoxicity, and patients with renal impairment may require dosage adjustments of drugs cleared renally, such as some opioids, and a balanced approach to fluid management.11
Sevoflurane with cisplatin makes non-small cell lung cancer cells more chemosensitive while making renal cell carcinoma cells more resistant to chemotherapy. Additionally, it was discovered that exposure to neoadjuvant chemotherapy and inhalation anesthetics aided in the course of the disease. These studies highlight the significance of understanding the type of cancer and, if feasible, its genetic components before choosing the best inhaled anesthetic for each patient’s treatment.3
Propofol and ketamine are two intravenous anesthetics that interact with chemotherapy medicines. Tyrosine kinase inhibitors and propofol used together in patients with chronic myeloid leukemia may have synergistic benefits. Propofol also has the ability to increase the apoptosis that cisplatin caused in cells. A study on ovarian cancer cells discovered that propofol caused the cancer cells to undergo apoptosis and improved the ability of both paclitaxel-sensitive and paclitaxel-resistant cancer cells to be killed by the drug. It was discovered that the high levels of transcription factor Slug expression in the paclitaxel-resistant cancer cells corresponded with greater chemoresistance. Administration of propofol resulted in noticeably reduced Slug levels, which raised the ovarian cancer cells’ chemosensitivity. Propofol has been shown to increase the tumor cells’ sensitivity to chemotherapy in several types of pancreatic tumors treated with gemcitabine.3
It has also been discovered that the usage of lidocaine and mitomycin-C can increase survival in specific tumor forms. A study using the triple-negative human breast cancer cell lines MCF-7 and MDA-MB-231 showed that lidocaine boosted the sensitizing effects of cisplatin. An increased apoptosis ratio and greater inhibitory impact of cisplatin were observed when it was coupled with lidocaine.3
Opioids used in conjunction with chemotherapy can lessen its cytotoxic effects. When cisplatin was administered, this effect was shown. It has been demonstrated that tramadol and flurbiprofen interfere with the cytotoxicity of cisplatin by affecting how gap junctions couple. The cytotoxicity of cisplatin is reduced as a result of the reduction in gap junction coupling.3
NSAIDS and certain chemotherapy drugs have been shown to work synergistically to increase the antitumor activity of such drugs, as well as to increase the rate of tolerance to chemotherapy and the overall response rate for cancer that is advanced in stage.3
One study found that dexamethasone lowered the sensitivity of triple-negative breast cancer cells when combined with a semi-synthetic analog of paclitaxel. Dexamethasone is used to treat a variety of illnesses, including cancer, and helps to manage the side effects of chemotherapy, radiation, and immunotherapies. Studies have revealed that the transcription of numerous genes, which encode for proteins that support epithelial cell survival during apoptosis, is directly stimulated through glucocorticoid receptor activation.3
Regarding regional anesthetics, it is important to be aware that a sizable proportion of patients, particularly those who have received cisplatin, may have a sub-clinical, undiagnosed neuropathy.5 However, Abcejo et al.15 showed that although peripheral neuropathy after systemic chemotherapy treatment is well described, they could not demonstrate whether systemic chemotherapy increases the risk of perioperative nerve injury after peripheral nerve blockade.
Anesthetic drugs can alter the immune response and modulate inflammation; however, the role of anesthesia in the outcome of cancer patients and in tumor recurrence and metastases has not yet been defined.16 The acute and long-term impacts of cancer treatment in clinical practice will be improved with more research into the optimal methods for practice.3,14 So far, there is insufficient evidence to warrant a change in clinical practice.17 According to Lacassie et al.18, anesthesia and analgesia techniques are important in the pain control and modulation of the inflammatory response, but do not appear to have a significant impact on the prognosis of some advanced cancer.
Since there may be changes in organ function (hemostasis, dysproteinaemia, hypoalbuminemia, cognitive dysfunction, immune response, compromised or difficult airway, mediastinal mass syndrome), independent of and/or in addition to the effect of anti-neoplastic agents, it is important to consider the underlying disease itself, nutritional status, and effects of concurrent therapy, such as radiation. In any event, patients who have anti-cancer chemotherapy should be closely examined for signs of subtly compromised pulmonary, renal, hepatic, or cardiac function.5
Conclusion
In conclusion, it is crucial for anesthetists to have a comprehensive understanding of the potential adverse effects of chemotherapy drugs and their interactions with anesthetics. Given the common occurrence of patients undergoing cancer therapy at various stages, anesthetists have a responsibility to be well-informed about how these medications can impact anesthesia administration. It is important to recognize that there may be clinical medication interactions that can increase both the toxicity of chemotherapeutic agents and the effects of anesthetics in patients who have received or are currently undergoing chemotherapy. Therefore, thorough preoperative evaluations that take into account the patient’s health in the context of prior anticancer therapy are essential. By acknowledging and addressing these potential interactions, anesthetists can ensure the safety and well-being of patients undergoing anesthesia in the context of cancer treatment.
References
| 1 |
Oprea AD, Russell RR, Russell KS, Abu-Khalaf M.
Chemotherapy agents with known cardiovascular side effects and their anesthetic implications.
J Cardiothorac Vasc Anesth. 2017;31(6):2206-2226.
|
| 2 | |
| 3 |
Watson J, Ninh MK, Ashford S, et al.
Anesthesia medications and interaction with chemotherapeutic agents.
Oncol Ther. 2021;9(1):121-138.
|
| 4 |
Page MJ, McKenzie JE, Bossuyt PM, et al.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
BMJ. 2021;372:n71.
|
| 5 |
Huettemann E, Sakka SG.
Anaesthesia and anti-cancer chemotherapeutic drugs.
Curr Opin Anaesthesiol. 2005;18(3):307-314.
|
| 6 | |
| 7 |
Huettemann E, Junker T, Chatzinikolaou KP, et al.
The influence of anthracycline therapy on cardiac function during anesthesia.
Anesth Analg. 2004;98(4):941-947.
|
| 8 |
Dana Oprea A.
Chemotherapy agents with known pulmonary side effects and their anesthetic and critical care implications.
J Cardiothorac Vasc Anesth. 2017;31(6):2227-2235.
|
| 9 |
Maracic L, Van Nostrand J, Beach D.
Update for nurse anesthetists.
AANA J. 2007;75(3):219-226.
|
| 10 |
Klein DS, Wilds PR.
Pulmonary toxicity of antineoplastic agents: anaesthetic and postoperative implications.
Can Anaesth Soc J. 1983;30(4):399-405.
|
| 11 |
Groenewold MD, Olthof CG, Bosch DJ.
Anaesthesia after neoadjuvant chemotherapy, immunotherapy or radiotherapy.
BJA Educ. 2022;22(1):12-19.
|
| 12 |
Kvolik S, Glavas-Obrovac L, Sakic K, Margaretic D, Karner I.
Anaesthetic implications of anticancer chemotherapy.
Eur J Anaesthesiol. 2003;20(11):859-871.
|
| 13 |
Cullen BF, Miller MG.
Drug interactions and anesthesia: a review.
Anesth Analg. 1979;58(5):413-423.
|
| 14 |
Zaniboni A, Prabhu S, Audisio RA.
Chemotherapy and anaesthetic drugs: too little is known.
Lancet Oncol. 2005;6(3):176-181.
|
| 15 |
Abcejo AS, Sviggum HP, Mauermann ML, et al.
Perioperative nerve injury after peripheral nerve block in patients with previous systemic chemotherapy.
Reg Anesth Pain Med. 2016;41(6):685-690.
|
| 16 |
Huitink JM, Teoh WHL.
Current cancer therapies—a guide for perioperative physicians.
Best Pract Res Clin Anaesthesiol. 2013;27(4):481-492.
|
| 17 |
Xuan W, Hankin J, Zhao H, Yao S, Ma D.
The potential benefits of the use of regional anesthesia in cancer patients.
Int J Cancer. 2015;137(12):2774-2784.
|
| 18 |
Lacassie HJ, Cartagena J, Brañes J, Assel M, Echevarría GC.
The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population.
Anesth Analg. 2013;117(3):653-660.
|