Abstract
Goal-directed fluid therapy (GDFT) has been proposed to optimize fluid management and reduce perioperative complications in the elderly. This meta-analysis evaluates the effects of intraoperative GDFT compared to conventional fluid therapy (CFT) on postoperative outcomes in elderly surgical patients. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline, randomized controlled trials (RCTs) were identified from six databases. Outcomes assessed included overall postoperative complications, 30-day mortality, hospital length of stay (LOS), and total fluid administered. Ten RCTs met the inclusion criteria, of which nine (n = 1,001) were included in the quantitative synthesis after excluding studies employing outdated protocols. GDFT significantly reduced overall complication rates (risk ratios [RR] = 0.80 [95% confidence interval (CI): 0.69–0.92];
Keywords
complications, elderly, goal-directed fluid therapy, intraoperative, meta-analysis
Introduction
Perioperative fluid management is a crucial aspect of surgical care, especially in elderly patients. Age-related physiological changes, including reduced renal function, hormonal changes, and alterations in body composition, make elderly patients more susceptible to the risks of fluid overload and hypovolemia during surgery. 1 Proper intraoperative fluid management is critical to improve overall surgical outcomes in this vulnerable population. One approach that has gained increasing attention in recent years is goal-directed fluid therapy (GDFT), a strategy that tailors fluid administration to individual patient needs based on real-time hemodynamic monitoring. 2
Although there is no consensus regarding the ideal “goals” for GDFT, various parameters and techniques have been evaluated. 2 Nevertheless, regardless of modality, previous individual studies that compared GDFT to conventional fluid therapy (CFT) have suggested that the former reduces complication rates, mortality, and hospital length of stay (LOS). 2 A couple of meta-analyses in the adult population undergoing gastrointestinal (GI) surgery confirm that morbidity was decreased in those receiving GDFT. 3,4 However, this finding was not repeated in another meta-analysis focusing on patients undergoing thoracic surgery. 5 Furthermore, only one meta-analysis reports LOS reduction, 3 emphasizing the variability of available evidence. GDFT has also been evaluated in pediatric patients. The meta-analysis by Kumba et al. 6 found a decrease in mortality, morbidity, and LOS in children receiving GDFT.
The elderly, given their higher risk for perioperative complications, may be particularly well-suited for GDFT. However, evidence regarding its effectiveness in this population is limited. A few randomized controlled trials (RCTs) have been conducted specifically in the elderly population, but there remains uncertainty regarding its efficacy as some studies show significant benefits while others report no major differences in morbidity. 7-10 Moreover, unlike the adult and pediatric populations, no meta-analysis has been performed in this population.
To address these knowledge gaps, this systematic review and meta-analysis aim to assess the effects of intraoperative GDFT in elderly patients undergoing surgery. By analyzing RCTs, this review will evaluate the impact of GDFT on key postoperative outcomes, including complication rates, length of hospital stay, and total intraoperative fluid volume administered. This analysis will provide a comprehensive overview of the current evidence and help inform clinical decision-making in perioperative fluid management for elderly patients.
Methods
Eligibility Criteria
Studies eligible for inclusion in the systematic review were selected based on the patient, intervention, comparison, outcome, and study design strategy framework. 11 Included patients were the elderly (aged ≥ 60 years old) 12,13 undergoing any surgical procedures. The intervention under review was GDFT with CFT as the comparator. The primary outcome measured was the occurrence of postoperative complications, defined as any adverse event identified during the post-surgical hospital stay, whereas the secondary outcomes measured included hospital LOS, 30-day mortality, and total intraoperative fluid volume administered. Lastly, only RCTs were included in this review. Studies in languages other than English or Indonesian; those without a clear description of the intraoperative fluid management protocol; those not reporting the primary outcome of interest; and those designed as observational studies, case reports, and non-randomized trials were excluded from the review.
For the meta-analysis, studies were excluded if they utilized monitoring parameters that are no longer recommended in modern GDFT protocols. Examples of such methods include, but are not limited to, fluid therapies guided by central venous pressure (CVP) and pulmonary capillary wedge pressure. 14-16 To ensure methodological consistency and relevance to practice, these studies were included in the qualitative synthesis but excluded from the quantitative meta-analysis.
Search Strategy
The search for relevant studies was conducted in October 2024 through six electronic databases (PubMed, Proquest, Scopus, Ovid Embase, Ovid MEDLINE, and Web of Science). MeSH terms and keywords derived from the terms “elderly”, “surgery”, “goal-directed fluid therapy”, “complications”, and “randomised controlled trials” were used. Boolean operators and truncation were incorporated to optimise the scope of search results. The detailed search strategy is presented in Table A1 in the Appendix. Additional studies were identified through manual hand-searching and reference tracing from relevant articles.
Study Screening and Selection
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. 17 Collected articles were imported into the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia), and duplicates were subsequently removed. 18 Two independent authors screened the identified studies against the inclusion criteria. Initially, the studies were screened according to their title and abstracts. Those who passed the initial screening process had their full text reviewed for inclusion in the final analysis. Discrepancies in decisions were settled by consensus. The screening process is summarized in Figure 1.
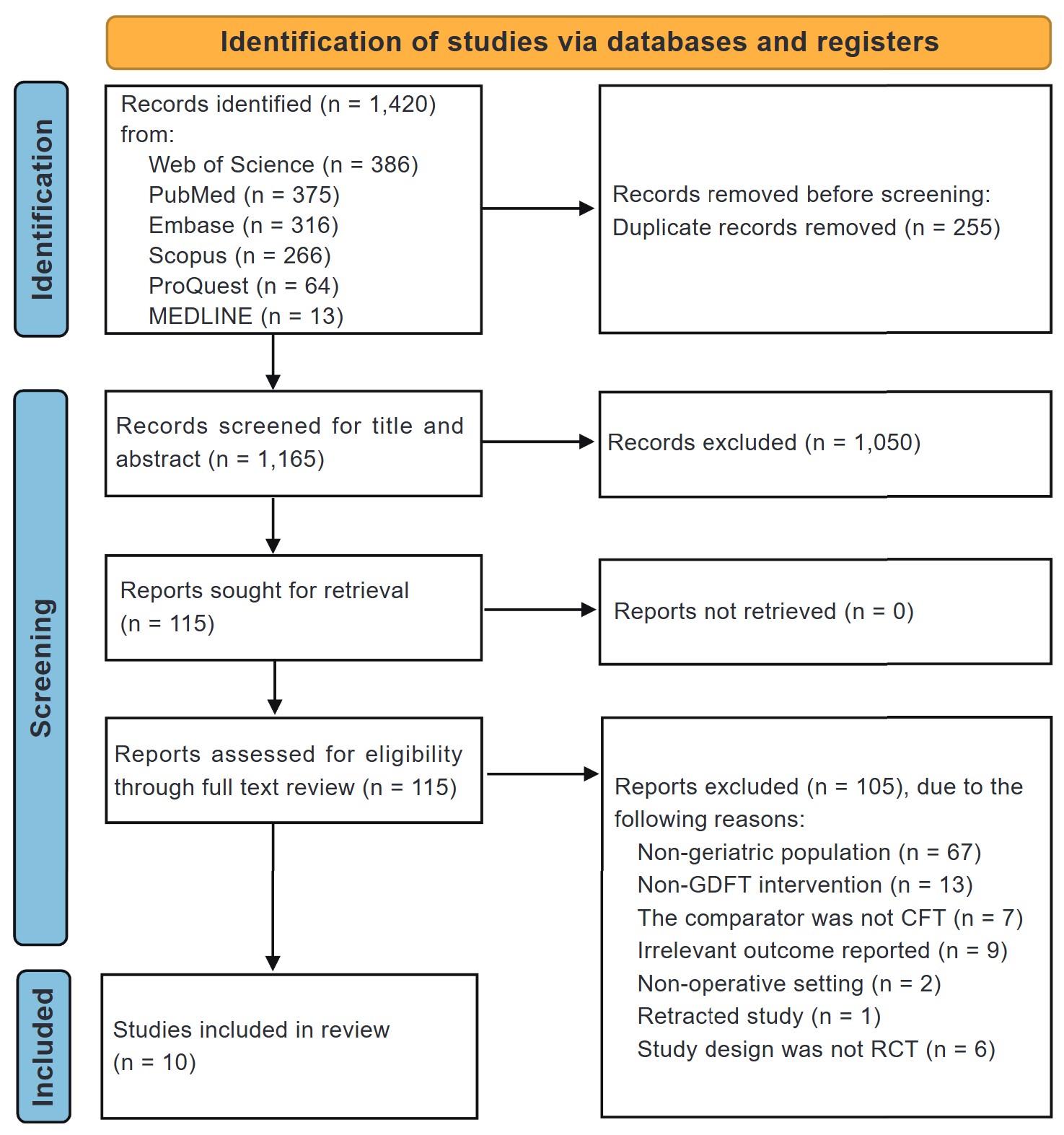
Download full-size image
Abbreviations: CFT, conventional fluid therapy; GDFT, goal-directed fluid therapy; RCT, randomized controlled trials.
Data Extraction
Two authors extracted the following data from the included studies: identifiers of each study (title, authors, year, and country), baseline population information (sample size, sex, age, type of surgery, and type of anesthesia), the specific GDFT parameters used, and relevant outcomes as previously mentioned. One author (JEFL) was tasked with data collection, and another (APM) checked the accuracy of the data extraction form afterwards.
Risk-of-Bias Assessment
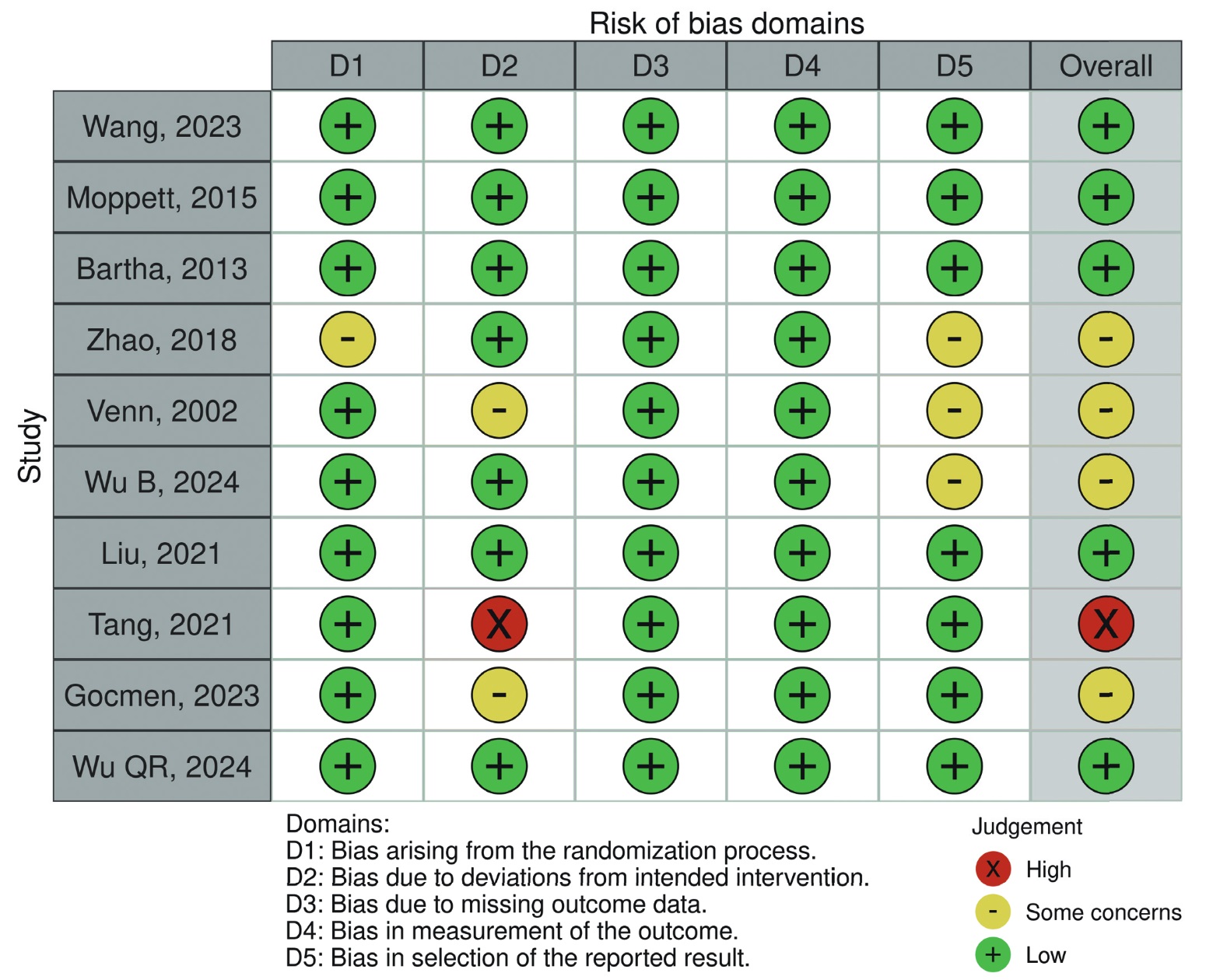
Two investigators independently assessed the risk of bias of included RCTs using the revised Cochrane tool for assessing the risk of bias in randomised trials (RoB). 19 Bias risk of the following aspects were categorised as either “low”, “some concerns”, or “high”: bias due to the randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of reported results. Only studies with all aspects appraised as “low” are considered to have an overall low risk of bias. Those with one or more aspects marked “high” are considered to be a high risk of bias, whereas the rest are considered to be some concerns for bias risks. 19 Any disagreements were resolved through consensus. Plots summarizing the bias risks of each study were then generated using the Risk-of-bias VISualization (robvis) tool. 20
Statistical Analysis
Extracted data were exported to the Review Manager software 5.4 (Cochrane Collaboration, Oxford, United Kingdom) for quantitative analysis. Continuous outcomes were analysed using the inverse variance method to obtain the effect estimate, i.e., the mean difference (MD), and their respective 95% confidence interval (CI). For dichotomous variables, risk ratios (RRs) and 95% CIs were obtained through the Mantel–Haenszel method. For studies reporting continuous variables as medians, ranges, and/or interquartile ranges, the available data is converted into means and standard deviations using the method developed by Wan et al. 21
The Cochran’s Q test and Higgins’ I
2
statistic were used to evaluate the included studies’ heterogeneity.
11,22
If the
Protocol Registration
A detailed protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) prior to the review (registration ID: CRD42024595378).
Results
Search Results
A total of 1,420 studies were obtained from the database search. After the removal of duplicates, 1,165 unique studies’ titles and abstracts were screened, and 1,050 articles were subsequently removed. All 115 remaining studies were retrieved for full text screening, and a further 105 were excluded due to reasons mentioned in Figure 1. In the end, 10 RCTs were included for quantitative synthesis. 7-10,24-29 The selection process is illustrated in the PRISMA flow diagram (Figure 1).
Characteristics of Included Studies
The 10 RCTs comprise a total of 1,061 patients, with 528 and 533 patients in the GDFT and CFT groups, respectively. Table 1 7-10,24-29 provides the baseline characteristics and summary of relevant findings from the included studies. The studies encompassed various types of surgery, including proximal femoral fracture repair (4 studies), GI surgery (5 studies), and esophagectomy (1 study). A variety of hemodynamic targets were used to guide GDFT. The most commonly used parameters were stroke volume variation (SVV) and cardiac index (CI). Other parameters included pulse pressure variation (PPV), stroke volume (SV), and CVP—the latter used in only one study. Pulse contour analysis was the most frequently employed device for GDFT, used in 7 out of 10 studies. Other devices included pleth variability index sensors and central venous catheter pressure transducers. Furthermore, the clinical benefits of GDFT varied across studies, some with contradicting results. The overall risk of bias is shown in Figure 2. Five of the included studies have a low risk of bias, 7,8,24,26,29 four studies have some concerns for bias risks, 9,10,25,28 , and one study was considered to be a high risk of bias. 27 A more detailed risk of bias assessment of individual studies is provided in Figure A1 in the Appendix.
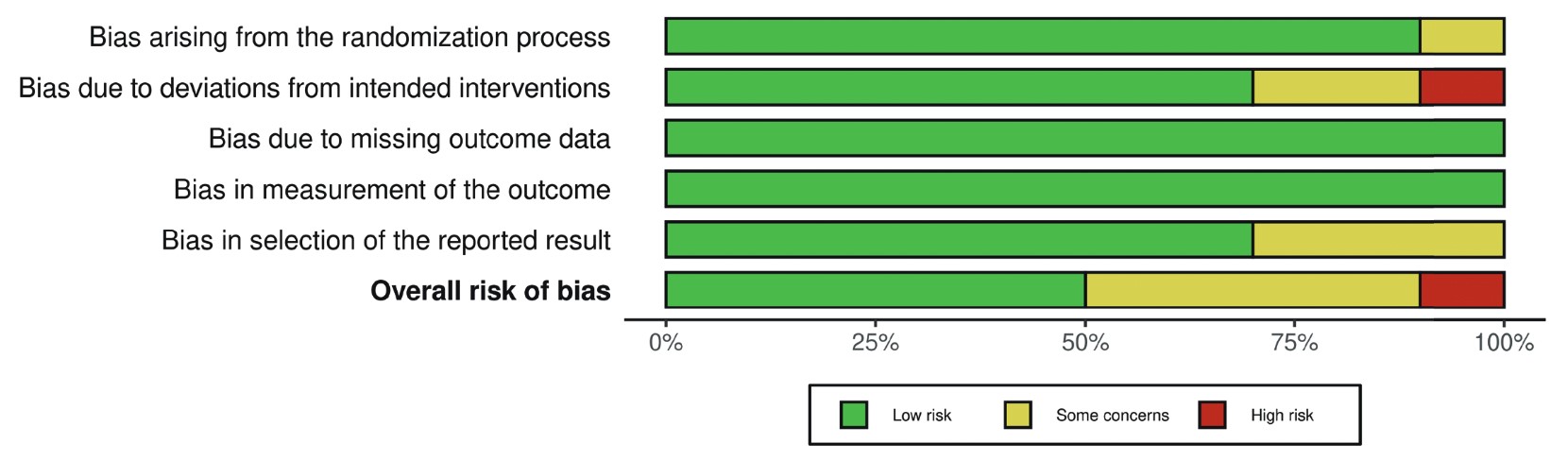
Download full-size image
Each risk of bias item is presented as percentage across all studies. Green, yellow, and red indicate a low risk of bias, some concerns for bias, and a high risk of bias, respectively.
|
Author, year, country |
Number of patients |
Mean age (years ± SD) |
Type of surgery |
Type of anesthesia |
GDFT goals |
Device for GDFT |
Summary of relevant outcomes |
|||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
GDFT |
CFT |
|
GDFT |
CFT |
|
|
|
|
|
|
Venn et al. 10 , 2002, UK |
31 |
29 |
85.50 ± 5.84 |
84.00 ± 9.13 |
Proximal femoral fracture repair |
General anesthesia |
CVP |
CVC connected to a pressure transducer |
Compared to CFT, GDFT: • increases total IV fluids • shows no difference in LOS, morbidity and mortality |
|
|
Bartha et al. 7 , 2013, Sweden |
29 |
25 |
86.00 ± 7.40 |
85.25 ± 7.89 |
Proximal femoral fracture repair |
Spinal anesthesia |
SV, DO 2 I |
Pulse contour analysis (LiDCO) |
Compared to CFT, GDFT: • decreases total IV fluids • shows no difference in complication rates, LOS, mortality. |
|
|
Moppett et al. 24 , 2015, UK |
51 |
63 |
83.63 ± 7.58 |
83.00 ± 6.47 |
Proximal femoral fracture repair |
Spinal anesthesia |
SV |
Pulse contour analysis (LiDCO) |
Compared to CFT, GDFT shows no difference in complication rates, LOS, mortality, total IV fluids |
|
|
Zhao et al. 25 , 2018, China |
44 |
44 |
67.77 ± 7.99 |
70.41 ± 8.99 |
Gastrointestinal surgery |
General anesthesia |
SVV |
Pulse contour analysis (Vigileo/ FloTrac) |
Compared to CFT, GDFT: • decreases total IV fluids • shows no difference in LOS, complication rates |
|
|
Liu et al. 26 , 2021, China |
60 |
60 |
68.30 ± 5.49 |
69.20 ± 6.55 |
Gastrointestinal surgery |
General anesthesia |
CI, SVV |
Pulse contour analysis (Vigileo/ FloTrac) |
Compared to CFT, GDFT: • decreases total IV fluids, complication rates • shows no difference in LOS, mortality |
|
|
Tang et al. 27 , 2021, China |
33 |
32 |
69 ± 3 |
70 ± 5 |
Esophagectomy |
General anesthesia |
SVV |
Pulse contour analysis (PiCCO) |
Compared to CFT, GDFT shows no difference in complication rates, total IV fluids, mortality, and LOS |
|
|
Göçmen et al. 28 , 2023, Turkey |
30 |
30 |
80 ± 8 |
80.0 ± 7.9 |
Proximal femoral fracture repair |
General anesthesia |
SVV, PPV, CI |
Pulse contour analysis (MostCare) |
Compared to CFT, GDFT: • decreases LOS • shows no difference in mortality, complication rates, total crystalloid volume |
|
|
Wang et al. 8 , 2023, China |
107 |
104 |
70.7 ± 5.4 |
70.7 ± 5.2 |
Gastrointestinal surgery |
General anesthesia |
PVI |
PVI sensor |
Compared to CFT, GDFT: • decreases total IV fluids • shows no difference in complication rates, mortality, LOS |
|
|
Wu et al. 29 , 2024, China |
58 |
56 |
70.2 ± 7.0 |
70.0 ± 7.5 |
Gastrointestinal surgery |
General anesthesia |
PPV |
Not mentioned |
Compared to CFT, GDFT: • decreases complication rates, total IV fluids, LOS |
|
|
Wu et al. 9 , 2024, China |
40 |
40 |
66.37 ± 4.85 |
64.87 ± 5.12 |
Gastrointestinal surgery |
General anesthesia |
CI, SV, SVV |
Pulse contour analysis (Vigileo/ FloTrac) |
Compared to CFT, GDFT: • decreases complication rates, total IV fluids • shows no difference in LOS |
|
Abbreviations: CFT, conventional fluid therapy; CI, cardiac index; CVC, central venous catheter; CVP, central venous pressure; DO2I, oxygen delivery index; GDFT, goal-directed fluid therapy; IV, intravenous; LOS, length of stay; PPV, pulse pressure variation; PVI, pleth variability index; RCT, randomized controlled trials; SD, standard deviation; SV, stroke volume; SVV, stroke volume variation.
Meta-Analysis of Outcomes
Of the 10 studies included in the systematic review, one study by Venn et al. 10 was excluded from the meta-analysis as it utilized a GDFT method based on CVP monitoring, which is no longer recommended in modern clinical practice.
Overall Complication Rates
Nine RCTs reported overall complication rates (n = 1,001).
7-9,24-29
The pooled RR was 0.80 (95% CI: 0.69–0.92;
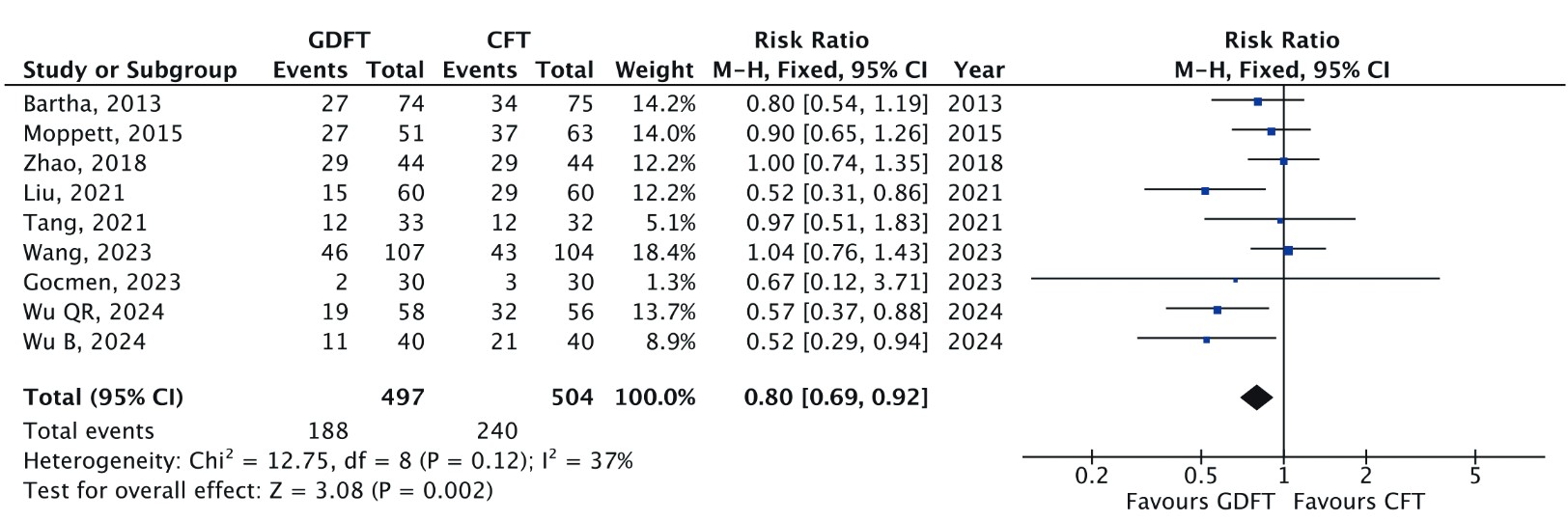
Download full-size image
A Mantel-Haenszel fixed effects model was used to calculate the risk ratio with a 95% confidence interval (CI).
Mortality Within 30 Days
Five RCTs reported 30-day mortality rates (n = 654).
7,8,24,26,28
Although the pooled analysis (Figure 4) showed no statistically significant difference between GDFT and CFT (RR = 0.42 [95% CI: 0.17, 1.04];
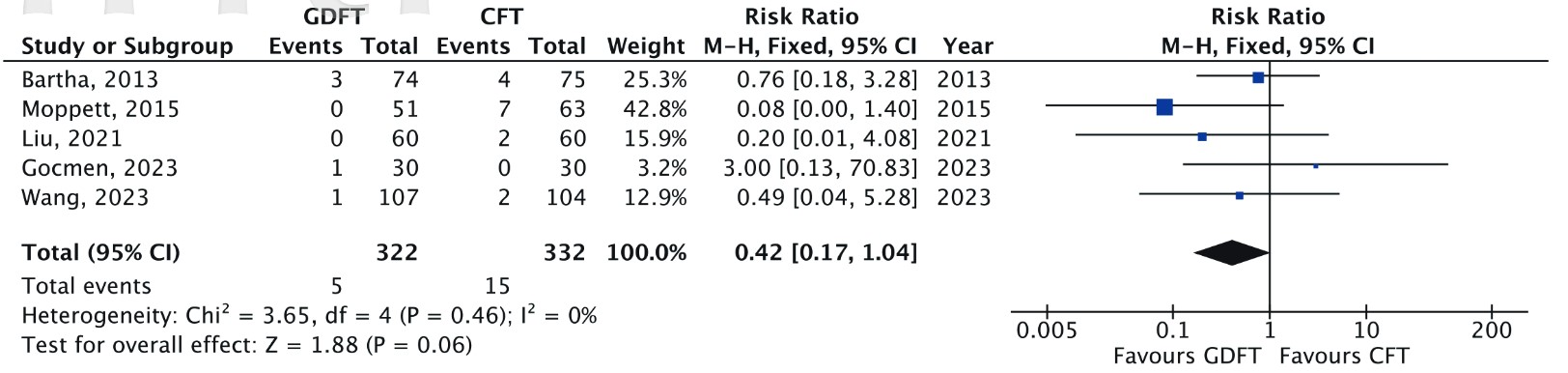
Download full-size image
A Mantel-Haenszel fixed effects model was used to calculate the risk ratio with a 95% confidence interval.
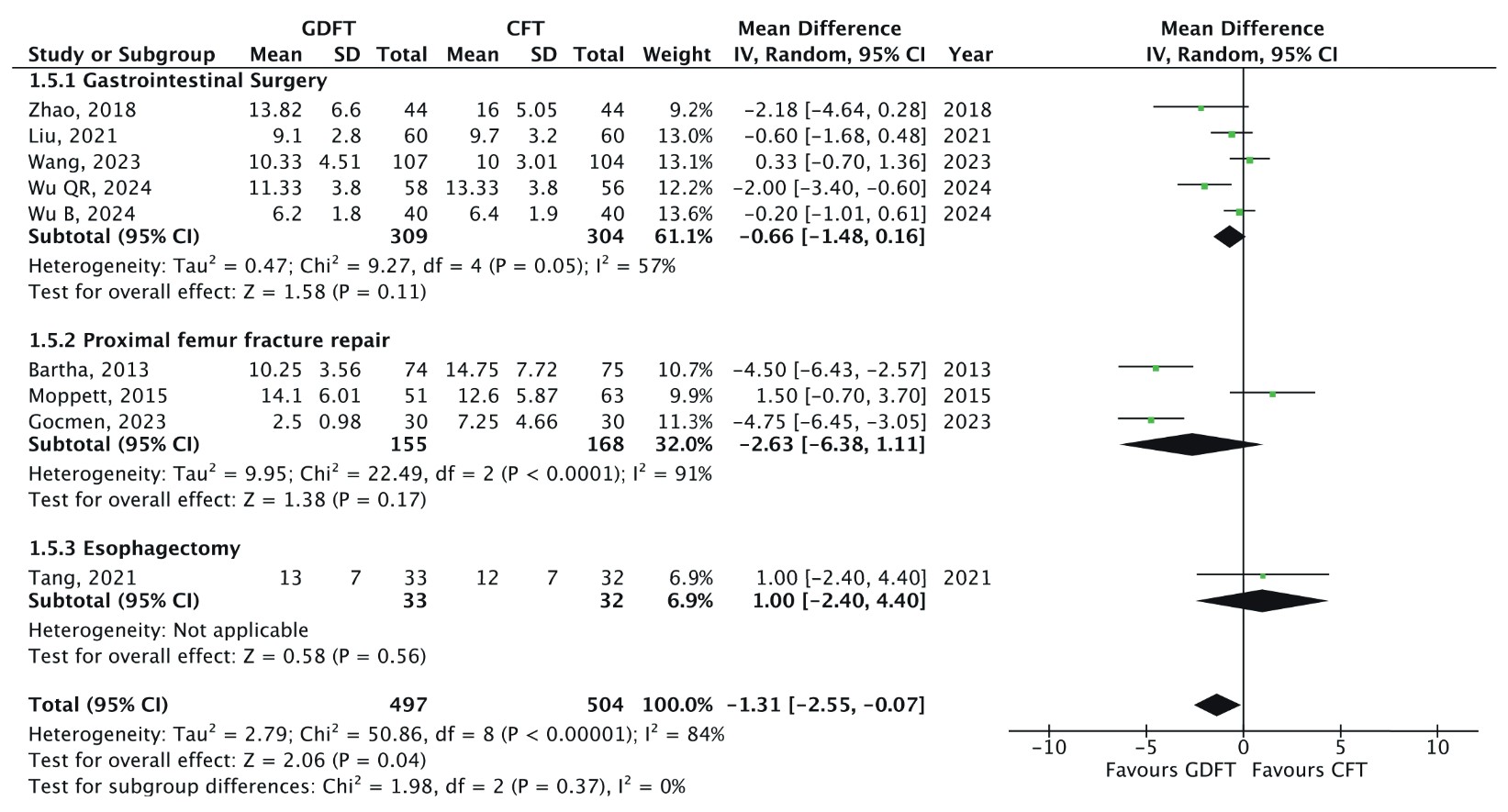
Hospital LOS
All nine RCTs reported hospital LOS (n = 1,001).
7-9,24-29
The pooled MD was –1.31 days (95% CI: –2.55 to –0.07;
Download full-size image
An inverse variance random effects model was used to calculate the mean difference with a 95% confidence interval (CI).
Subgroup analysis based on surgical type did not yield statistically significant differences, and no significant differences were detected between subgroups. Additionally, the level of heterogeneity remained unchanged despite subgroup stratification. Sensitivity analysis, performed by sequentially removing individual studies, did not identify a single study as the primary source of heterogeneity. However, further analysis showed that the pooled results lost statistical significance when specific studies were excluded.
7,9,25,26,28,29
This suggests that while the overall trend favours GDFT, the robustness of the findings is influenced by specific trials within the meta-analysis.
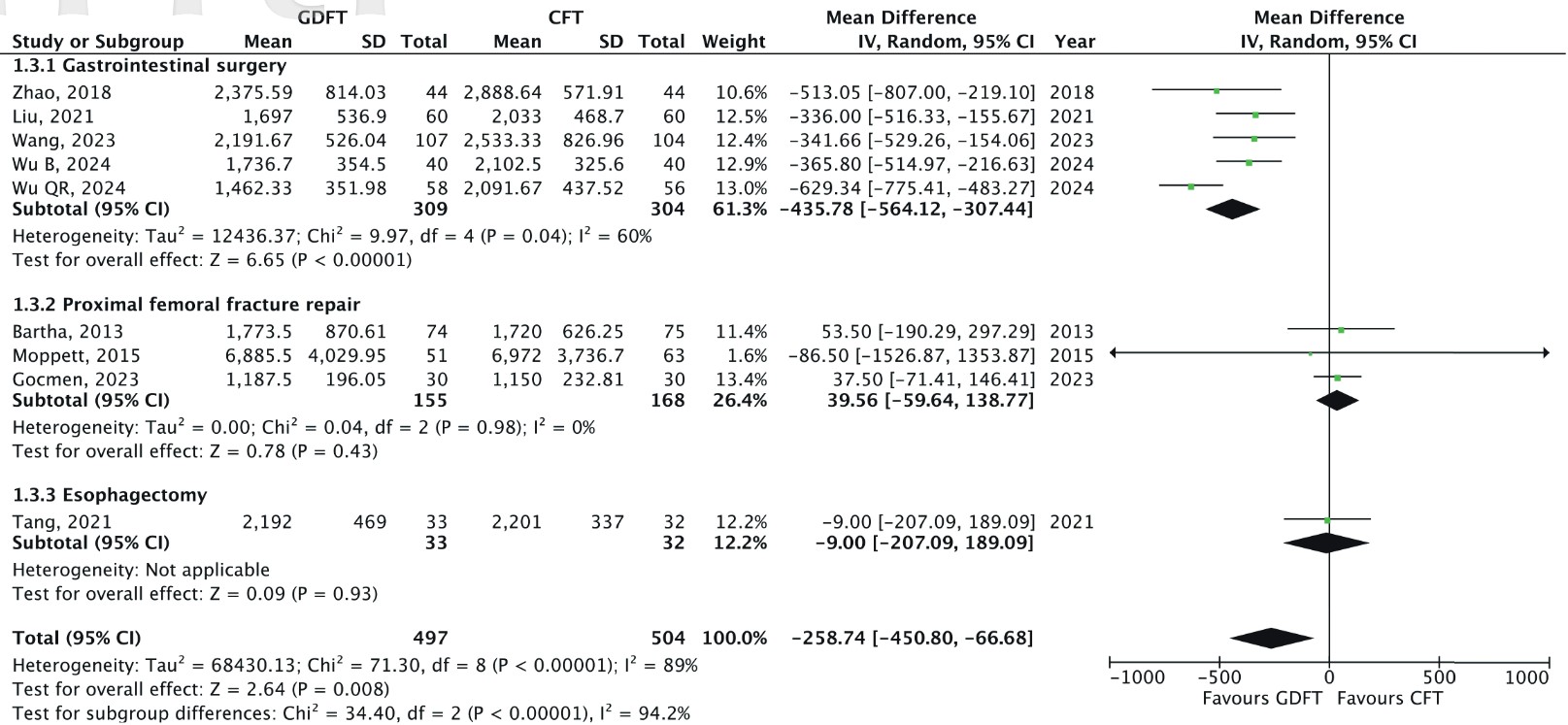
Total Intraoperative Fluid Volume
All nine RCTs reported total intraoperative fluid administered (n = 1,001).
7-9,24-29
The pooled MD was –258.74 mL (95% CI: –450.86 to –66.68;
Download full-size image
An inverse variance random effects model was used to calculate the mean difference with a 95% confidence interval.
Subgroup analysis based on the type of surgery revealed distinct patterns. In GI surgery, GDFT was associated with significantly less intraoperative fluids (MD: –435.78, 95% CI: –564.12 to –307.44;
Sensitivity analysis revealed that the exclusion of Wu et al. 29 from the GI surgery subgroup reduced its heterogeneity to I 2 = 0%, indicating that this study contributed to the observed variability. Further, the pooled results remained statistically significant throughout the sensitivity analysis. Thus, the findings were deemed robust.
Discussion
The present systematic review examined the effects of intraoperative GDFT compared to CFT across 10 RCTs involving a total of 1,061 elderly patients. Of these, nine studies, comprising 1,001 patients, were included in the meta-analysis. The pooled results indicate that GDFT is associated with a reduction in overall postoperative complication rates, hospital LOS, and total fluid administered. However, no statistically significant difference was observed in mortality
outcomes.
The included studies varied in their choice of hemodynamic targets and monitoring devices, ranging from dynamic parameters such as SVV, CI, and PPV, to the relatively outdated CVP, which has limited predictive value for fluid responsiveness. 2,14,15 The majority of studies employed pulse contour analysis systems, reflecting their increasing role in modern GDFT protocols due to their ability to continuously track hemodynamic parameters and related variables in a minimally invasive manner. 2
Furthermore, recent studies increasingly emphasize the use of pulse contour analysis to monitor dynamic parameters such as SVV and CI. These dynamic targets have been more consistently associated with improved clinical outcomes in elderly surgical patients when compared to traditional static measures like CVP. Static parameters have repeatedly been shown to be unreliable predictors of fluid responsiveness. 2,30 This is because fluid responsiveness depends on the patient’s position on the Frank–Starling curve, which cannot be determined from a single pressure or volume reading. 30 Their accuracy is further compromised by external factors such as intrathoracic pressure, intraabdominal pressure, ventilator settings, venous tone, and cardiac function. 14,31 In contrast, dynamic parameters assess real-time changes during the respiratory cycle, which better predict the patient’s responsiveness to volume expansion and, thus, offer a more clinically relevant approach to guide fluid management. 30
Benefits of Intraoperative GDFT in the Elderly
A key finding of this meta-analysis is the significant reduction in postoperative complications with the use of GDFT. This result aligns with previous studies conducted in both adult and pediatric populations, 3,4,6 supporting the concept that GDFT improves patient outcomes by tailoring fluid administration based on advanced hemodynamic monitoring rather than relying on standard static parameters. The elderly population, in particular, stands to benefit greatly from GDFT due to their reduced physiological reserve and increased susceptibility to fluid imbalances, whether in the form of overload (which can precipitate pulmonary edema and heart failure) or under-resuscitation (which can lead to organ hypoperfusion). 1,32 By utilizing dynamic indices, GDFT enables clinicians to administer fluids only when the patient is likely to be “fluid responsive”, thus minimizing the risk of fluid-related complications.
Despite the observed benefit, a moderate degree of heterogeneity (I2
= 37%;
In addition to reducing complications, GDFT was associated with a lower volume of total fluids administered, highlighting its efficiency in avoiding unnecessary fluids. This finding is consistent with a meta-analysis by Han et al. 5 in the adult population. Further, the decrease in fluids aligns with the principle of “fluid optimization”, suggesting that less fluid can be utilised without compromising hemodynamic stability. Avoiding excessive fluid in the elderly is paramount, as they are less capable of compensating for volume overload due to decreased renal and cardiovascular function. 1,33
However, a high degree of heterogeneity was noted in the overall analysis (I2 = 89%), suggesting variability across studies. Subgroup analysis revealed that the effect of GDFT on intraoperative fluid volume varied by surgery type. In GI surgery, GDFT was associated with significantly lower intraoperative fluid administration, though heterogeneity within this subgroup remained moderate (I2 = 60%). Notably, sensitivity analysis within this subgroup indicated that excluding the study by Wu et al. 29 reduced heterogeneity to I2 = 0%, suggesting that their study contributed to the observed variability. Several factors may explain this. First, Wu et al. 29 did not specify the tool used to measure their GDFT parameters, introducing potential inconsistencies in measurement and interpretation. Second, their study relied solely on PPV-guided fluid therapy, whereas the other included studies predominantly used SVV. Given that SVV has been shown to have superior diagnostic accuracy in predicting fluid responsiveness compared to PPV, 34,35 this methodological difference may have contributed to the heterogeneity observed in the primary analysis.
In contrast to GI surgeries, studies focusing on proximal femoral fracture repair and esophagectomy did not show significant differences in fluid administration and exhibited low heterogeneity (I 2 = 0%). Multiple reasons may explain these intergroup differences. GI surgeries typically have longer operative durations, providing a greater window for real-time hemodynamic monitoring and fluid titration, which may enhance the effectiveness of GDFT. Additionally, unlike many orthopedic procedures, such as proximal femoral fracture repair, which are often performed under spinal anesthesia, GI surgeries are usually conducted under general anesthesia. This suggests that GDFT may be more useful in titrating fluid volume in patients undergoing the latter. Importantly, sensitivity analyses showed that the overall results remained statistically significant even after sequential exclusion of individual studies, supporting the robustness of the observed effect.
Another important benefit observed was the reduction in hospital LOS by more than one day in patients receiving GDFT. This can be explained by the reduction of postoperative complications mentioned previously. Multiple studies have found that complication rates increase hospital LOS, including in the geriatric population. 36-38 Interestingly, previous meta-analyses in adults have reported variable results regarding the effects of GDFT on LOS. 3-5 The discrepancy may indicate that the elderly are a unique group who are at risk of prolonged postoperative LOS 39 and, thus, GDFT is more effective in reducing it in this population.
Nonetheless, the high degree of heterogeneity observed (I2 = 84%) suggests variability across studies. Subgroup analysis by surgical type did not resolve this issue, and sensitivity analyses failed to pinpoint a single outlier study driving the variability. This indicates that multiple factors may contribute to the observed variability, such as differences in differences in patient demographics, surgical complexity, and perioperative management strategies. Additionally, the pooled results lost statistical significance when specific studies were excluded, 7,9,25,26,28,29 highlighting that the robustness of our findings is influenced by individual trials within the meta-analysis.
The benefits of preventing postoperative complications expand beyond shorter hospital stays. Research has shown that elderly patients who experience postsurgical complications take longer to recover to their baseline preoperative functional status, 40 are more dissatisfied with their care, 41 and are more prone to readmissions. 42,43 Moreover, the cost of hospital care increases when patients experience postoperative complications. 36,44 Overall, these evidence magnifies the benefits of GDFT through prevention of surgical complications in the elderly.
Lack of Significant Effect on Mortality
Our analysis did not detect a statistically significant difference in 30-day mortality between the GDFT and CFT groups, which is consistent with findings from previous meta-analyses in adult populations. 3,4 Several reasons may account for this finding. First, postoperative mortality, especially in the elderly, is a complex and multifactorial outcome that may not be directly influenced by fluid management alone. Patient comorbidities, the type and urgency of surgery, baseline organ function, and the postoperative care environment can all significantly affect survival rates. 45-47 Second, the effect of GDFT on mortality might be limited due to the relatively short follow-up periods of most studies included in the meta-analysis and, thus, may not capture longer-term survival benefits.
Interestingly, although the overall pooled estimate did not reach statistical significance, sensitivity analysis revealed that excluding the study by Göçmen et al.
28
resulted in a significant difference in mortality favoring GDFT (
Strengths of the Meta-Analysis
One of the key strengths of this meta-analysis is that, to the best of the authors’ knowledge, it is the first to focus specifically on the impact of intraoperative GDFT in the elderly population. Given that older adults are increasingly undergoing surgical procedures and are at heightened risk for perioperative complications, this focus fills a critical gap in the existing literature. Second, the analysis was based on a thorough and systematic search of multiple databases as recommended by the literature, 48 which reduces the risks of publication bias. Third, the study adhered to PRISMA guidelines and used a registered protocol, adding to the transparency and reproducibility of the methodology and findings. Fourth, by exclusively including RCTs, this analysis ensures high methodological rigor. In addition, subgroup and sensitivity analyses were conducted to explore potential sources of heterogeneity and to assess the robustness of the findings. This provides a more comprehensive interpretation of the results and strengthens confidence in the observed associations. Finally, this meta-analysis excluded studies incorporating outdated methods such as CVP-guided fluid therapy, 14,15 ensuring that the findings remain applicable to current clinical practice and contemporary hemodynamic monitoring strategies.
Limitations
The current meta-analysis has several limitations that must be acknowledged. First, only English-language studies were included, which may introduce language bias and potentially exclude relevant data published in other languages. However, a previous study has suggested that language-based restrictions have little effect on effect estimates. 49
Second, notable heterogeneity was observed in the analyses of hospital LOS and total intraoperative fluid volume administered. Although sensitivity analyses were conducted, residual heterogeneity remained, indicating that variability across studies could not be fully explained by individual outliers. Subgroup analysis by type of surgery reduced heterogeneity in intraoperative fluid volume, but did not resolve heterogeneity in LOS, suggesting that additional unmeasured factors, such as differences in GDFT protocols, postoperative care practices, and/or discharge criteria, may contribute.
Third, the risk of bias remains a concern. While most studies had a low risk of bias, four trials were judged to have some concerns, and one was rated as high risk, which may affect the reliability of their findings.
Finally, the surgical procedures included in this meta-analysis are limited to orthopaedic surgeries, GI surgeries, and one esophagectomy, restricting the generalizability of our conclusions to broader surgical populations. Hence, extrapolating these findings to other perioperative settings should be approached with caution.
Future Directions
While the present meta-analysis highlights the potential benefits of intraoperative GDFT in reducing postoperative complications, hospital LOS, and fluid administration in the elderly population, several areas warrant further investigation. First, given the significant heterogeneity observed in our analysis, future studies should focus on optimizing the consistency of GDFT protocols. Standardized guidelines for hemodynamic targets and monitoring technologies may help reduce variability across studies and enhance comparability.
Additionally, future research should consider including studies published in other languages and address different surgical contexts to provide a more comprehensive understanding of GDFT’s impacts. Further, given the notable risk of bias in a few included studies, high-quality, large-scale RCTs are needed to strengthen the evidence base. Lastly, there is a need for research on long-term outcomes, such as postoperative functional recovery, quality of life, and readmission rates, to determine whether the short-term benefits of GDFT extend beyond the immediate postoperative period.
Conclusion
This meta-analysis demonstrates that intraoperative GDFT significantly reduces postoperative complication rates and hospital LOS in elderly patients, underscoring its potential to optimize perioperative care in this high-risk population. A reduction in total intraoperative fluid volume was also observed; however, this effect appeared to be driven primarily by studies involving GI surgery. The substantial heterogeneity in certain outcomes and the presence of bias in some included studies warrant cautious interpretation. Future high-quality trials are needed to standardize GDFT protocols and evaluate their efficacy across a broader range of surgical procedures to better inform clinical practice.
References
| 1 |
El-Sharkawy AM, Sahota O, Maughan RJ, Lobo DN.
The pathophysiology of fluid and electrolyte balance in the older adult surgical patient.
Clin Nutr. 2014;33(1):6-13.
|
| 2 |
Kendrick JB, Kaye AD, Tong Y, et al.
Goal-directed fluid therapy in the perioperative setting.
J Anaesthesiol Clin Pharmacol. 2019;35(Suppl 1):S29-S34.
|
| 3 |
Rollins KE, Lobo DN.
Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials.
Ann Surg. 2016;263(3):465-476.
|
| 4 |
Xu C, Peng J, Liu S, et al.
Goal-directed fluid therapy versus conventional fluid therapy in colorectal surgery: a meta analysis of randomized controlled trials.
Int J Surg. 2018;56:264-273.
|
| 5 |
Han S, Wu X, Li P, He K, Li J.
The impact of goal-directed fluid therapy on postoperative pulmonary complications in patients undergoing thoracic surgery: a systematic review and meta-analysis.
J Cardiothorac Surg. 2024;19(1):60.
|
| 6 |
Kumba C, Willems A, Querciagrossa S, et al.
A systematic review and meta-analysis of intraoperative goal directed fluid and haemodynamic therapy in children and postoperative outcome.
J Emerg Med Critical Care. 2019;5(1):9.
|
| 7 |
Bartha E, Arfwedson C, Imnell A, Fernlund ME, Andersson LE, Kalman S.
Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture.
Br J Anaesth. 2013;110(4):545-553.
|
| 8 |
Wang Y, Zhang Y, Zheng J, et al.
Intraoperative pleth variability index-based fluid management therapy and gastrointestinal surgical outcomes in elderly patients: a randomised controlled trial.
Perioper Med (Lond). 2023;12(1):16.
|
| 9 |
Wu B, Guo Y, Min S, Xiong Q, Zou L.
Postoperative cognitive dysfunction in elderly patients with colorectal cancer: a randomized controlled study comparing goal-directed and conventional fluid therapy.
Open Med (Wars). 2024;19(1):20240930.
|
| 10 |
Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P.
Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures.
Br J Anaesth. 2002;88(1):65-71.
|
| 11 |
Higgins JPT, Thomas J, Chandler J, et al.
Cochrane Handbook for Systematic Reviews of Interventions
.
|
| 12 |
Dugarova E.
Ageing, Older Persons and the 2030 Agenda for Sustainable Development
.
|
| 13 |
World Health Organization.
Tackling Abuse of Older People: Five Priorities for the United Nations Decade of Healthy Ageing (2021–2030)
.
1st ed.
|
| 14 |
Eskesen TG, Wetterslev M, Perner A.
Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness.
Intensive Care Med. 2016;42(3):324-332.
|
| 15 |
Marik PE, Cavallazzi R.
Does the central venous pressure predict fluid responsiveness?
Crit Care Med. 2013;41(7):1774-1781.
|
| 16 | |
| 17 |
Page MJ, McKenzie JE, Bossuyt PM, et al.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
BMJ. 2021;372:n71.
|
| 18 |
McKeown S, Mir ZM.
Considerations for conducting systematic reviews: evaluating the performance of different methods for de-duplicating references.
Syst Rev. 2021;10(1):38.
|
| 19 |
Sterne JAC, Savović J, Page MJ, et al.
RoB 2: a revised tool for assessing risk of bias in randomised trials.
BMJ. 2019;366:l4898.
|
| 20 |
McGuinness LA, Higgins JPT.
Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments.
Res Synth Methods. 2021;12(1):55-61.
|
| 21 |
Wan X, Wang W, Liu J, Tong T.
Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range.
BMC Med Res Methodol. 2014;14:135.
|
| 22 |
Higgins JPT, Thompson SG.
Quantifying heterogeneity in a meta-analysis.
Stat Med. 2002;21(11):1539-1558.
|
| 23 |
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR.
A basic introduction to fixed-effect and random-effects models for meta-analysis.
Res Synth Methods. 2010;1(2):97-111.
|
| 24 |
Moppett IK, Rowlands M, Mannings A, Moran CG, Wiles MD, NOTTS Investigators.
LiDCO-based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: a randomized trial and systematic review.
Br J Anaesth. 2015;114(3):444-459.
|
| 25 |
Zhao G, Peng P, Zhou Y, Li J, Jiang H, Shao J.
The accuracy and effectiveness of goal directed fluid therapy in plateau-elderly gastrointestinal cancer patients: a prospective randomized controlled trial.
Int J Clin Exp Med. 2018;11(8):8516-8522.
|
| 26 |
Liu X, Zhang P, Liu MX, Ma JL, Wei XC, Fan D.
Preoperative carbohydrate loading and intraoperative goal-directed fluid therapy for elderly patients undergoing open gastrointestinal surgery: a prospective randomized controlled trial.
BMC Anesthesiol. 2021;21(1):157.
|
| 27 |
Tang W, Qiu Y, Lu H, Xu M, Wu J.
Stroke volume variation-guided goal-directed fluid therapy did not significantly reduce the incidence of early postoperative complications in elderly patients undergoing minimally invasive esophagectomy: a randomized controlled trial.
Front Surg. 2021;8:794272.
|
| 28 |
Göçmen D, Köksal C, Abitağaoğlu S, Yildirim Arzu.
Comparison of the effects of intraoperative goal directed and conventional fluid management on the inferior Vena cava collapsibility index and postoperative complications in geriatric patients operated from proximal femoral nail surgery.
Türk Geriatr Derg. 2023;26(1):37-47.
|
| 29 |
Wu QR, Zhao ZZ, Fan KM, Cheng HT, Wang B.
Pulse pressure variation guided goal-direct fluid therapy decreases postoperative complications in elderly patients undergoing laparoscopic radical resection of colorectal cancer: a randomized controlled trial.
Int J Colorectal Dis. 2024;39(1):33.
|
| 30 |
Monnet X, Marik PE, Teboul JL.
Prediction of fluid responsiveness: an update.
Ann Intensive Care. 2016;6(1):111.
|
| 31 |
Sondergaard S, Parkin G, Aneman A.
Central venous pressure: we need to bring clinical use into physiological context.
Acta Anaesthesiol Scand. 2015;59(5):552-560.
|
| 32 |
Doherty M, Buggy DJ.
Intraoperative fluids: how much is too much?
Br J Anaesth. 2012;109(1):69-79.
|
| 33 |
Brandstrup B, Møller AM.
The challenge of perioperative fluid management in elderly patients.
Curr Anesthesiol Rep. 2019;9(4):406-413.
|
| 34 |
Hofer CK, Müller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A.
Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting.
Chest. 2005;128(2):848-854.
|
| 35 |
Khwannimit B, Bhurayanontachai R.
Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation.
Eur J Anaesthesiol. 2012;29(2):64-69.
|
| 36 |
Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA.
Association of postoperative complications with hospital costs and length of stay in a tertiary care center.
J Gen Intern Med. 2006;21(2):177-180.
|
| 37 |
Sieber FE, Barnett SR.
Preventing postoperative complications in the elderly.
Anesthesiol Clin. 2011;29(1):83-97.
|
| 38 |
Pirson M, Dehanne F, Van den Bulcke J, Leclercq P, Martins D, De Wever A.
Evaluation of cost and length of stay, linked to complications associated with major surgical procedures.
Acta Clin Belg. 2018;73(1):40-49.
|
| 39 |
Polanczyk CA, Marcantonio E, Goldman L, et al.
Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery.
|
| 40 |
Tahiri M, Sikder T, Maimon G, et al.
The impact of postoperative complications on the recovery of elderly surgical patients.
Surg Endosc. 2016;30(5):1762-1770.
|
| 41 |
Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM.
Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients.
Br J Anaesth. 2000;84(1):6-10.
|
| 42 |
Lawson EH, Hall BL, Louie R, et al.
Association between occurrence of a postoperative complication and readmission: implications for quality improvement and cost savings.
Ann Surg. 2013;258(1):10-18.
|
| 43 |
Tian M, Wang Z, Zhu Y, Tian Y, Zhang K, Li X.
Incidence, causes, and risk factors of unplanned readmissions in elderly patients undergoing hip fracture surgery: an observational study.
Clin Interv Aging. 2023;18:317-326.
|
| 44 |
Yuan F, Essaji Y, Belley-Cote EP, et al.
Postoperative complications in elderly patients following pancreaticoduodenectomy lead to increased postoperative mortality and costs.
Int J Surg. 2018;60:204-209.
|
| 45 |
Heriot AG, Tekkis PP, Smith JJ, et al.
Prediction of postoperative mortality in elderly patients with colorectal cancer.
Dis Colon Rectum. 2006;49(6):816-824.
|
| 46 |
Tzeng CWD, Cooper AB, Vauthey JN, Curley SA, Aloia TA.
Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients.
HPB (Oxford). 2014;16(5):459-468.
|
| 47 |
Vester-Andersen M, Lundstrøm LH, Møller MH, et al.
Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study.
Br J Anaesth. 2014;112(5):860-870.
|
| 48 |
Harari MB, Parola HR, Hartwell CJ, Riegelman A.
Literature searches in systematic reviews and meta-analyses: a review, evaluation, and recommendations.
Journal of Vocational Behavior. 2020;118:103377.
|
| 49 |
Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M.
Direction and impact of language bias in meta-analyses of controlled trials: empirical study.
Int J Epidemiol. 2002;31(1):115-123.
|
Appendix
Download full-size image
Green, yellow, and red indicate a low risk of bias, some concerns for bias, and a high risk of bias, respectively.
|
Database |
Search Strategy |
Hits |
|---|---|---|
|
PubMed |
1. ((((((((((((((((fluid therapies[MeSH Terms]) OR (fluid therapy[MeSH Terms])) OR (parenteral infusion[MeSH Terms])) OR (parenteral infusions[MeSH Terms])) OR (goal-directed fluid therapy[Title/Abstract])) OR (precision fluid management[Title/Abstract])) OR (targeted fluid therapy[Title/Abstract])) OR (hemodynamic-guided fluid therapy[Title/Abstract])) OR (optimized fluid therapy[Title/Abstract])) OR (individualized fluid management[Title/Abstract])) OR (individualized fluid therapy[Title/Abstract])) OR (fluid optimization[Title/Abstract])) OR (tailored fluid therapy[Title/Abstract])) 2. ((((((((((((((((((complications[MeSH Terms]) OR (intraoperative complication[MeSH Terms])) OR (intraoperative complications[MeSH Terms])) OR (complication, peroperative[MeSH Terms])) OR (complications, peroperative[MeSH Terms])) OR (surgical injuries[MeSH Terms])) OR (surgical injury[MeSH Terms])) OR (postoperative complication[MeSH Terms])) OR (postoperative complications[MeSH Terms])) OR (dehiscence, surgical wound[MeSH Terms])) OR (infection, surgical wound[MeSH Terms])) OR (cardiovascular complications[Title/Abstract])) OR (respiratory complications[Title/Abstract])) OR (renal complications[Title/Abstract])) OR (gastrointestinal complications[Title/Abstract])) OR (morbidity[Title/Abstract])) OR (adverse events[Title/Abstract])) OR (perioperative complications[Title/Abstract]))) 3. ((((((((((((((elderly[MeSH Terms]) OR (elderly, frail[MeSH Terms])) OR (geriatrics[MeSH Terms])) OR (aged[MeSH Terms])) OR (frail older adult[MeSH Terms])) OR (aged, 80 and over[MeSH Terms])) OR (centenarian[MeSH Terms])) OR (nonagenarian[MeSH Terms])) OR (octogenarian[MeSH Terms])) OR (elderly[Title/Abstract])) OR (aged[Title/Abstract])) OR (senior[Title/Abstract])) OR (geriatric[Title/Abstract])) OR (older[Title/Abstract]))) 4. ((((((((((((surgery[MeSH Terms]) OR (general surgery[MeSH Terms])) OR (surgical procedures, operative[MeSH Terms])) OR (operations[MeSH Terms])) OR (operative procedure[MeSH Terms])) OR (invasive procedures[MeSH Terms])) OR (surgery[Title/Abstract])) OR (surgical procedure[Title/Abstract])) OR (operation[Title/Abstract])) OR (operative procedure[Title/Abstract])) OR (surgical technique[Title/Abstract])) OR (invasive procedure[Title/Abstract]))) 5. ((((((((((((((((allocation, random[MeSH Terms]) OR (clinical trials, randomized[MeSH Terms])) OR (controlled clinical trials, randomized[MeSH Terms])) OR (randomized controlled trials as topic[MeSH Terms])) OR (clinical trial[MeSH Terms])) OR (clinical trial as topic[MeSH Terms])) OR (clinical trial, phase 1[MeSH Terms])) OR (clinical trial, phase 2[MeSH Terms])) OR (clinical trial, phase 3[MeSH Terms])) OR (clinical trial, phase 4[MeSH Terms])) OR (controlled clinical trial[MeSH Terms])) OR (controlled clinical trials as topic[MeSH Terms])) OR (randomized clinical trial[Title/Abstract])) OR (randomized trial[Title/Abstract])) OR (randomized controlled clinical trials[Title/Abstract])) OR (clinical trial[Title/Abstract])) 6. #1 AND #2 AND #3 AND #4 AND #5 |
375 |
|
Web of Science |
1. ((((((((((ALL=(fluid therap*)) OR ALL=(parenteral infusion*)) OR ALL=(goal-directed fluid therapy)) OR ALL=(precision fluid managementt)) OR ALL=(targeted fluid therapy)) OR ALL=(hemodynamic-guided fluid therapy)) OR ALL=(optimized fluid therapy)) OR ALL=(individualized fluid management)) OR ALL=(individualized fluid therapy)) OR ALL=(fluid optimization)) OR ALL=(tailored fluid therapy) 2. (((((((((((((ALL=(complication*)) OR ALL=(intraoperative complication*)) OR ALL=(peroperative complication*)) OR ALL=(surgical injur*)) OR ALL=(postoperative complication*)) OR ALL=(surgical wound dehiscence)) OR ALL=(infection*)) OR ALL=(cardiovascular complication*)) OR ALL=(respiratory complication*)) OR ALL=(renal complication*)) OR ALL=(gastrointestinal complication*)) OR ALL=(morbidity)) OR ALL=(adverse event*)) OR ALL=(perioperative complication*) 3. ((((((((ALL=(elderly)) OR ALL=(frail elderly)) OR ALL=(geriatric*)) OR ALL=(aged)) OR ALL=(frail older adult)) OR ALL=(centenarian)) OR ALL=(nonagenarian)) OR ALL=(octogenarian)) OR ALL=(old*) 4. ((((ALL=(surgery)) OR ALL=(general surgery)) OR ALL=(surgical procedure*)) OR ALL=(operation*)) OR ALL=(invasive procedure) 5. ((((ALL=(randomised controlled trial*)) OR ALL=(randomized controlled trial*)) OR ALL=(randomised clinical trial)) OR ALL=(randomized controlled trial*)) OR ALL=(clinical trial) 6. #1 AND #2 AND #3 AND #4 AND #5 |
368 |
|
ProQuest |
1. "fluid therapy" OR "goal-directed fluid therapy" OR "precision fluid management" OR "targeted fluid therapy" OR "hemodynamic-guided fluid therapy" OR "optimized fluid therapy" OR "individualized fluid management" OR "individualized fluid therapy" OR "fluid optimization" OR "tailored fluid therapy" 2. "complication" OR "intraoperative complication" OR "peroperative complication" OR "surgical injury" OR "postoperative complication" OR "surgical wound dehiscence" OR "infection" OR "cardiovascular complication" OR "respiratory complication" OR "renal complication" OR "gastrointestinal complication" OR morbidity OR "adverse event" OR "perioperative complication" 3. elderly OR "frail elderly" OR geriatric OR aged OR "frail older adult" OR centenarian OR nonagenarian OR octogenarian OR old 4. surgery OR "general surgery" OR "surgical procedure" OR operation OR "invasive procedure" 5. "randomised controlled trial" OR "randomized controlled trial" OR "randomised clinical trial" OR "randomized controlled trial" OR "clinical trial" 6. "conventional fluid therapy" OR "standard fluid therapy" OR "routine fluid management" OR "fixed-rate fluid therapy" OR "traditional fluid therapy" OR "standard care fluid administration" OR "non-guided fluid management" OR "usual care fluid therapy" 7. #1 AND #2 AND #3 AND #4 AND #5 AND #6 |
64 |
|
Embase |
1. "fluid therapy" OR "goal-directed fluid therapy" OR "precision fluid management" OR "targeted fluid therapy" OR "hemodynamic-guided fluid therapy" OR "optimized fluid therapy" OR "individualized fluid management" OR "individualized fluid therapy" OR "fluid optimization" OR "tailored fluid therapy" |
316 |
|
2. "complication" OR "intraoperative complication" OR "peroperative complication" OR "surgical injury" OR "postoperative complication" OR "surgical wound dehiscence" OR "infection" OR "cardiovascular complication" OR "respiratory complication" OR "renal complication" OR "gastrointestinal complication" OR morbidity OR "adverse event" OR "perioperative complication" 3. elderly OR "frail elderly" OR geriatric OR aged OR "frail older adult" OR centenarian OR nonagenarian OR octogenarian OR old 4. surgery OR "general surgery" OR "surgical procedure" OR operation OR "invasive procedure" 5. "randomised controlled trial" OR "randomized controlled trial" OR "randomised clinical trial" OR "randomized controlled trial" OR "clinical trial" 6. #1 AND #2 AND #3 AND #4 AND #5 |
316 |
|
|
Scopus |
1. ((((((((((((((((INDEXTERMS("fluid therapies")) OR (INDEXTERMS("fluid therapy"))) OR (INDEXTERMS("parenteral infusion"))) OR (INDEXTERMS("parenteral infusions"))) OR (TITLE-ABS("goal-directed fluid therapy"))) OR (TITLE-ABS("precision fluid management"))) OR (TITLE-ABS("targeted fluid therapy"))) OR (TITLE-ABS("hemodynamic-guided fluid therapy"))) OR (TITLE-ABS("optimized fluid therapy"))) OR (TITLE-ABS("individualized fluid management"))) OR (TITLE-ABS("individualized fluid therapy"))) OR (TITLE-ABS("fluid optimization"))) OR (TITLE-ABS("tailored fluid therapy"))) 2. ((((((((((((((((((INDEXTERMS(complications)) OR (INDEXTERMS("intraoperative complication"))) OR (INDEXTERMS("intraoperative complications"))) OR (INDEXTERMS("complication, peroperative"))) OR (INDEXTERMS("complications, peroperative"))) OR (INDEXTERMS("surgical injuries"))) OR (INDEXTERMS("surgical injury"))) OR (INDEXTERMS("postoperative complication"))) OR (INDEXTERMS("postoperative complications"))) OR (INDEXTERMS("dehiscence, surgical wound"))) OR (INDEXTERMS("infection, surgical wound"))) OR (TITLE-ABS("cardiovascular complications"))) OR (TITLE-ABS("respiratory complications"))) OR (TITLE-ABS("renal complications"))) OR (TITLE-ABS("gastrointestinal complications"))) OR (TITLE-ABS(morbidity))) OR (TITLE-ABS("adverse events"))) OR (TITLE-ABS("perioperative complications")))) 3. ((((((((((((((INDEXTERMS(elderly)) OR (INDEXTERMS("elderly, frail"))) OR (INDEXTERMS(geriatrics))) OR (INDEXTERMS(aged))) OR (INDEXTERMS("frail older adult"))) OR ("aged, 80" AND INDEXTERMS(over))) OR (INDEXTERMS(centenarian))) OR (INDEXTERMS(nonagenarian))) OR (INDEXTERMS(octogenarian))) OR (TITLE-ABS(elderly))) OR (TITLE-ABS(aged))) OR (TITLE-ABS(senior))) OR (TITLE-ABS(geriatric))) OR (TITLE-ABS(older)))) 4. ((((((((((((INDEXTERMS(surgery)) OR (INDEXTERMS("general surgery"))) OR (INDEXTERMS("surgical procedures, operative"))) OR (INDEXTERMS(operations))) OR (INDEXTERMS("operative procedure"))) OR (INDEXTERMS("invasive procedures"))) OR (TITLE-ABS(surgery))) OR (TITLE-ABS("surgical procedure"))) OR (TITLE-ABS(operation))) OR (TITLE-ABS("operative procedure"))) OR (TITLE-ABS("surgical technique"))) OR (TITLE-ABS("invasive procedure")))) |
266 |
|
5. ((((((((((((((((INDEXTERMS("allocation, random")) OR (INDEXTERMS("clinical trials, randomized"))) OR (INDEXTERMS("controlled clinical trials, randomized"))) OR (INDEXTERMS("randomized controlled trials as topic"))) OR (INDEXTERMS("clinical trial"))) OR (INDEXTERMS("clinical trial as topic"))) OR (INDEXTERMS("clinical trial, phase 1"))) OR (INDEXTERMS("clinical trial, phase 2"))) OR (INDEXTERMS("clinical trial, phase 3"))) OR (INDEXTERMS("clinical trial, phase 4"))) OR (INDEXTERMS("controlled clinical trial"))) OR (INDEXTERMS("controlled clinical trials as topic"))) OR (TITLE-ABS("randomized clinical trial"))) OR (TITLE-ABS("randomized trial"))) OR (TITLE-ABS("randomized controlled clinical trials"))) OR (TITLE-ABS("clinical trial"))) 6. #1 AND #2 AND #3 AND #4 AND #5 |
266 |
|
|
Medline |
1. (((((((((((((((((exp "fluid therapies"/) OR (exp "fluid therapy"/)) OR (exp "parenteral infusion"/)) OR (exp "parenteral infusions"/)) OR ("goal-directed fluid therapy".tw.)) OR ("precision fluid management".tw.)) OR ("targeted fluid therapy".tw.)) OR ("hemodynamic-guided fluid therapy".tw.)) OR ("optimized fluid therapy".tw.)) OR ("individualized fluid management".tw.)) OR ("individualized fluid therapy".tw.)) OR ("fluid optimization".tw.)) OR ("tailored fluid therapy".tw.)) 2. ((((((((((((((((((exp complications/) OR (exp "intraoperative complication"/)) OR (exp "intraoperative complications"/)) OR (exp "complication, peroperative"/)) OR (exp "complications, peroperative"/)) OR (exp "surgical injuries"/)) OR (exp "surgical injury"/)) OR (exp "postoperative complication"/)) OR (exp "postoperative complications"/)) OR (exp "dehiscence, surgical wound"/)) OR (exp "infection, surgical wound"/)) OR ("cardiovascular complications".tw.)) OR ("respiratory complications".tw.)) OR ("renal complications".tw.)) OR ("gastrointestinal complications".tw.)) OR (morbidity.tw.)) OR ("adverse events".tw.)) OR ("perioperative complications".tw.))) 3. ((((((((((((((exp elderly/) OR (exp "elderly, frail"/)) OR (exp geriatrics/)) OR (exp aged/)) OR (exp "frail older adult"/)) OR ("aged, 80" AND exp over/)) OR (exp centenarian/)) OR (exp nonagenarian/)) OR (exp octogenarian/)) OR (elderly.tw.)) OR (aged.tw.)) OR (senior.tw.)) OR (geriatric.tw.)) OR (older.tw.))) 4. ((((((((((((exp surgery/) OR (exp "general surgery"/)) OR (exp "surgical procedures, operative"/)) OR (exp operations/)) OR (exp "operative procedure"/)) OR (exp "invasive procedures"/)) OR (surgery.tw.)) OR ("surgical procedure".tw.)) OR (operation.tw.)) OR ("operative procedure".tw.)) OR ("surgical technique".tw.)) OR ("invasive procedure".tw.))) 5. ((((((((((((((((exp "allocation, random"/) OR (exp "clinical trials, randomized"/)) OR (exp "controlled clinical trials, randomized"/)) OR (exp "randomized controlled trials as topic"/)) OR (exp "clinical trial"/)) OR (exp "clinical trial as topic"/)) OR (exp "clinical trial, phase 1"/)) OR (exp "clinical trial, phase 2"/)) OR (exp "clinical trial, phase 3"/)) OR (exp "clinical trial, phase 4"/)) OR (exp "controlled clinical trial"/)) OR (exp "controlled clinical trials as topic"/)) OR ("randomized clinical trial".tw.)) OR ("randomized trial".tw.)) OR ("randomized controlled clinical trials".tw.)) OR ("clinical trial".tw.))) 6. (((((((("conventional fluid therapy".tw.) OR ("standard fluid therapy".tw.)) OR ("routine fluid management".tw.)) OR ("fixed-rate fluid therapy".tw.)) OR ("traditional fluid therapy".tw.)) OR ("standard care fluid administration".tw.)) OR ("non-guided fluid management".tw.)) OR ("usual care fluid therapy".tw.)) 7. #1 AND #2 AND #3 AND #4 AND #5 AND #6 |
13 |