Abstract
Background
Dexamethasone is widely used in anesthesia practise as prophylaxis for postoperative nausea and vomiting (PONV). Our aims were to evaluate the postoperative glycemic profile after a single dose of intraoperative dexamethasone in non-diabetic and diabetic patients and to evaluate the PONV.
Methods
This double-blinded, randomized, controlled study was done for 6 months from June to December 2024 in a tertiary care hospital after obtaining ethical committee clearance and CTRI registration. Patients were allocated to one of four groups: nondiabetics receiving saline, nondiabetics receiving dexamethasone, diabetics receiving saline, and diabetics receiving dexamethasone. The study drug or saline control was administered at the induction of anesthesia. Blood sugar values, two hours after dexamethasone/placebo administration as well as fasting blood sugar and postprandial blood sugar on postoperative day 1, 2 were taken as primary endpoints. PONV was assessed in the first 24 hours after surgery.
Results
The rise in blood glucose levels was higher in the group receiving dexamethasone compared to saline in both diabetics and
nondiabetics
(
Conclusions
Dexamethasone can be considered as prophylaxis for PONV in well controlled diabetics and nondiabetics despite the increase in blood glucose levels as a hyperglycemic response (blood glucose value 180 mg/dL) to a single dose was not observed in our study.
Keywords
blood glucose, dexamethasone, diabetic patients, glycaemic response, perioperative
Introduction
Postoperative nausea and vomiting (PONV) is a common problem encountered in the postoperative period which can hamper the quality of recovery and make the recovery period a harrowing experience for the patient. 1 There are numerous risk factors contributing to PONV including type of anesthesia and surgery performed. 2 Several antiemetics are recommended, and dexamethasone is one of the commonly used drugs for the same.
Dexamethasone is widely used in anesthesia practise as it has many beneficial effects which are mainly due to its anti-inflammatory actions. 3-5 Although chronic use of steroids can cause poor wound healing and surgical site infection, a single dose of dexamethasone in the perioperative period will not do so as shown by previous studies. 6 Dexamethasone may increase blood glucose levels in the postoperative period and cause untoward side effects and several studies have been conducted to assess the increase in blood glucose levels after single dose administration in the perioperative period, and contradictory results have been obtained. 7-9 This increase may be due to increased insulin resistance and diminished function of beta cells of pancreas. 9 The rise in blood glucose could vary between diabetic and non-diabetic patients. 10,11
Our aims were to evaluate not only the postoperative glycaemic profile after a single dose of intraoperative dexamethasone in non-diabetic and diabetic patients but also the PONV.
Methods
Population
This double-blinded, randomized, controlled study was done over a period of 6 months from June to December 2024 in a tertiary care hospital after obtaining ethical committee clearance and CTRI registration (CTRI/2024/06/068738).
Inclusion criteria: 18- to 70-year-old American Society of Anaesthesiologists (ASA) I, II and patients undergoing general anesthesia for elective laparoscopic surgeries.
Exclusion criteria: (1) preoperative use of steroids, (2) peptic ulcer, (3) pregnancy, (4) malignancy, (5) immunosuppression, (6) severe renal impairment (creatinine > 1.6) and liver impairment (liver enzyme > 2 times normal), and (7) pre-operative blood glucose levels greater than 11.1 mmol/L.
Written informed consent was taken from willing patients posted for elective laparoscopic surgery under general anesthesia. Preoperative blood sugar values were documented along with age, gender, body mass index, type of surgery, and ASA grading. All patients followed NPO (Nil per oral) guidelines of our institution which is 8 hours for solid foods and 2 hours for clear liquids. All patient received injection. Pantoprazole 40 mg 30 minutes before induction.
Double blinding was ensured by using preloaded 2 mL syringe of dexamethasone of 4 mg/mL dilution (labelled A) and saline (labelled B), prepared by an anesthesia resident unaware of the allocation, after the opening of a sealed opaque envelope containing the randomized assignment.
Patients were allocated to one of four groups: nondiabetics receiving saline, nondiabetics receiving dexamethasone, diabetics receiving saline, and diabetics receiving dexamethasone.
The study drug or saline control was administered at the induction of anesthesia in the operating room, along with premedication before starting preoxygenation by anesthesiology consultant who was blinded to the group. No dextrose-containing solution was administered during the study. In the operating theater, after attaching standard ASA monitors, anesthesia was induced with intravenous propofol after premedication with intravenous glycopyrrolate, midazolam, ondansetron 4 mg, and fentanyl. Muscle relaxation was achieved with atracurium 0.5 mg/kg, and endotracheal intubation was done. Anesthesia was maintained with a mixture of oxygen and nitrous oxide with isoflurane and intermittent doses of atracurium. At the end of surgery, patients were extubated after adequate reversal of neuromuscular blockade. As per institutional protocol, patients were encouraged to begin postoperative oral feeding 6 hours after extubation, provided there were no other surgical or anesthesia-related contraindications.
For diabetic patients, intraoperative blood glucose monitoring was done as per protocol. All blood glucose levels were measured using a calibrated glucometer, SD code free (SD Biosensor, Inc., Thailand). The blood glucose measurements were obtained from finger-prick capillary blood samples, by an anesthesiology resident blinded to the study group. The total amount of insulin administered for the treatment of perioperative hyperglycemia was recorded. The standard institutional protocol is to start variable rate insulin infusion when the blood glucose level is more than 180 mg/dL in the perioperative period.
Blood sugar values, two hours after dexamethasone/placebo administration, as well as fasting blood sugar (FBS) and postprandial blood sugar (PPBS) on postoperative day 1, 2 were taken as primary endpoints.
PONV in the first 24 hours was assessed using a 4-point verbal descriptive scale, which consists of score 0 = no PONV; score 1 = mild PONV: patient complains of nausea but refuses antiemetic treatment; score 2 = moderate PONV: patient complains of nausea and needs antiemetic treatment; score 3 = severe PONV. Patients who experienced PONV in the recovery area were treated with intravenous metoclopramide (10 mg).
Sample size was estimated by using the difference in mean FBS between nondiabetics who received dexamethasone and nondiabetics who received placebo from the study by Peter et al. 12 . Using the values at 95% confidence limit and 80% power, sample size of 33 was obtained in each group by using the formula (Sample size (N) = 2SD 2 (Zα/2 + Z β) 2 / d 2 ) and Med calc sample size software.
Statistical Analysis
Data was entered into Microsoft excel data sheet and was analyzed using SPSS 22 version software (IBM SPSS Statistics, Somers, NY, USA) and Epi-info version 7.2.1 (CDC Atlanta) software. Categorical data was represented in the form of frequencies and proportions. Chi-square test was used as test of significance for qualitative data. Continuous data was represented as mean
±
standard deviation. Normality of the continuous data, was tested by Kolmogorov–Smirnov test and the Shapiro–Wilk test. ANOVA (Analysis of Variance) was the test of significance to identify the mean difference between more than two groups for quantitative data. Graphical representation of data: MS Excel and MS word was used to obtain various types of graphs such as Box plot and line diagram. The
Results
A total of 132 patients participated in our study (Figure 1). Table 1 shows the demographic data of both diabetics and nondiabetics. In the data, 90.9 % of patients in nondiabetics were ASA I, and 9.1 % were ASA II with comorbidities other than diabetes. All the patients in diabetic group were ASA II. There was a significant difference in age between the diabetic and nondiabetics group. The mean age was higher in the diabetics group which was as expected (Table 2).
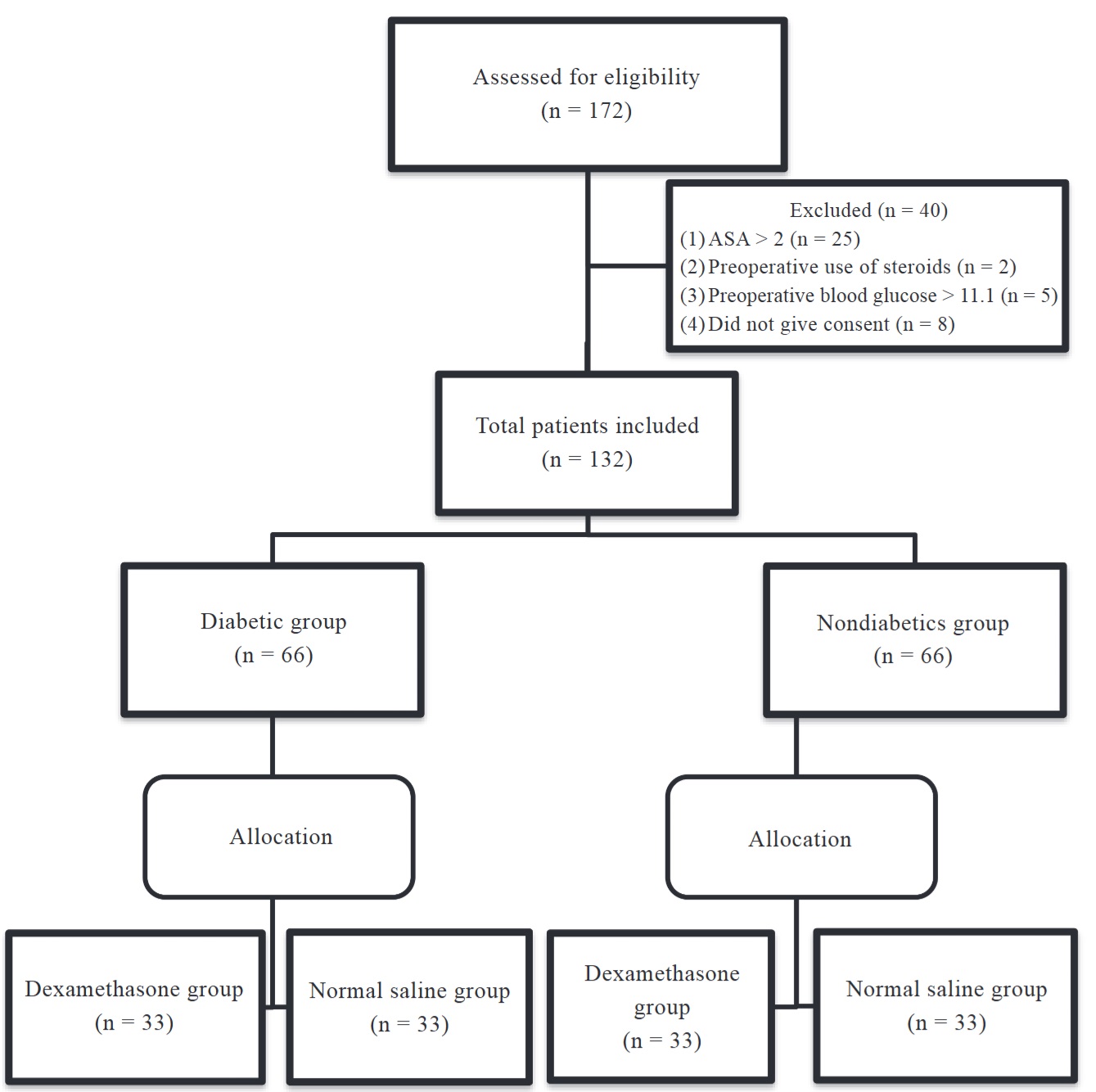
Download full-size image
Abbreviation: ASA, American Society of Anaesthesiologists.
|
Characteristic |
|
||
|---|---|---|---|
|
Nondiabetic group |
Diabetic group |
||
|
Age, mean (SD) |
39.94 (10.30) |
45.33 (9.29) |
0.002 * |
|
Gender (M/F) number |
11/55 |
22/44 |
0.027 * |
|
BMI, mean (SD) |
25.88 (2.80) |
25.96 (2.28) |
0.852 |
|
Duration (hours), mean (SD) |
2.5 (0.33) |
2.6 (0.37) |
0.1028 |
Abbreviations: BMI, body mass index; F, female; M, male; SD, standard deviation.
|
Variable |
Saline |
Dexamethasone |
|
|---|---|---|---|
|
Nondiabetic group |
|||
|
Age (mean ± SD) |
38.52 ± 11.01 |
41.36 ± 9.48 |
0.26 |
|
Gender (F%) |
87.9 |
78.8 |
0.32 |
|
BMI (mean ± SD) |
24.73 ± 2.47 |
27.03 ± 2.66 |
0.004 |
|
Surgery duration (mean ± SD) |
2.14 ± 0.21 |
2.39 ± 0.4 |
0.002 |
|
Diabetic group |
|||
|
Age (mean ± SD) |
44.27 ± 6.76 |
46.39 ± 11.29 |
0.35 |
|
Gender (F%) |
75.8 |
57.6 |
0.9 |
|
BMI (mean ± SD) |
25.59 ± 2.30 |
26.33 ± 2.23 |
0.18 |
|
Surgery duration (mean ± SD) |
2.71 ± 0.42 |
2.81 ± 0.32 |
0.27 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Tables 3 and 4 show the blood sugar values at different time points in nondiabetics and diabetics, respectively. The mean HbA1C taken preoperatively was comparable between the saline group and the dexamethasone group in both diabetics and nondiabetics. The rise in blood glucose levels was higher in the group receiving dexamethasone compared to saline in both diabetics and nondiabetics (
|
Variable |
Nondiabetics with dexamethasone |
Nondiabetics with saline |
|
|||
|---|---|---|---|---|---|---|
|
Mean ± SD |
95% CI |
Mean ± SD |
95% CI |
|||
|
Hba1c |
5.30 ± 0.18 |
[5.24, 5.37] |
5.22 ± 0.16 |
[5.17, 5.28] |
0.066 |
|
|
Preoperative RBS |
126.55 ± 9.70 |
[123.11, 129.98] |
125.67 ± 8.20 |
[122.76, 128.58] |
0.692 |
|
|
Preoperative FBS |
101.94 ± 10.16 |
[98.34, 105.54] |
106.12 ± 10.99 |
[102.23, 110.02] |
0.113 |
|
|
2nd hour glucose |
137.12 ± 5.59 |
[135.14, 139.10] |
125.61 ± 4.99 |
[123.84, 127.37] |
< 0.001 * |
|
|
8th hour glucose |
143.58 ± 6.05 |
[141.43, 145.72] |
130.91 ± 4.72 |
[129.24, 132.58] |
< 0.001 * |
|
|
POD1 FBS |
120.61 ± 5.33 |
[118.72, 122.49] |
114.61 ± 6.68 |
[112.24, 116.97] |
< 0.001 * |
|
|
POD1 PPBS |
137.94 ± 4.38 |
[136.39, 139.49] |
128.48 ± 6.76 |
[126.09, 130.88] |
< 0.001 * |
|
|
POD2 FBS |
115.79 ± 6.04 |
[113.65, 117.93] |
111.64 ± 7.87 |
[108.85, 114.43] |
0.019 * |
|
|
POD2 PPBS |
141.06 ± 4.09 |
[139.61, 142.51] |
132.12 ± 6.58 |
[129.79, 134.45] |
< 0.001 * |
|
Abbreviations: CI, confidence interval; FBS, fasting blood sugar; Hbalc, glycated hemoglobin; POD1/2, postoperative day one/two; PPBS, post prandial blood sugar; RBS, random blood sugar value; SD, standard deviation.
|
Variable |
Diabetics with dexamethasone |
Diabetics with saline |
|
|||
|---|---|---|---|---|---|---|
|
Mean ± SD |
95 % CI |
Mean ± SD |
95% CI |
|||
|
Hba1c |
6.99 ± 0.99 |
[6.64, 7.34] |
6.98 ± 0.39 |
[6.84, 7.12] |
0.914 |
|
|
Preoperative RBS |
140.15 ± 13.39 |
[135.40, 144.90] |
132.91 ± 11.73 |
[128.75, 137.07] |
0.658 |
|
|
Preoperative FBS |
112.55 ± 9.28 |
[109.26, 115.84] |
113.00 ± 11.59 |
[108.84, 117.16] |
0.862 |
|
|
2nd hour glucose |
149.06 ± 3.70 |
[147.75, 150.37] |
129.76 ± 4.20 |
[128.27, 131.25] |
< 0.001 * |
|
|
8th hour glucose |
156.09 ± 4.96 |
[154.33, 157.85] |
135.09 ± 4.28 |
[133.57, 136.61] |
< 0.001 * |
|
|
POD1 FBS |
133.03 ± 5.64 |
[131.03, 135.03] |
125.12 ± 5.23 |
[123.27, 126.98] |
< 0.001 * |
|
|
POD1 PPBS |
150.79 ± 4.03 |
[149.36, 152.22] |
143.94 ± 4.83 |
[142.20, 145.65] |
< 0.001 * |
|
|
POD2 FBS |
130.64 ± 6.07 |
[128.49, 132.79] |
124.42 ± 4.33 |
[122.89, 125.96] |
< 0.001 * |
|
|
POD2 PPBS |
151.27 ± 4.55 |
[149.66, 152.89] |
147.18 ± 3.89 |
[145.80, 148.56] |
< 0.001 * |
|
Abbreviations: CI, confidence interval; FBS, fasting blood sugar; HbAlc, glycated hemoglobin; POD1/2, postoperative day one/two; PPBS, post prandial blood sugar; RBS, random blood sugar value; SD, standard deviation.
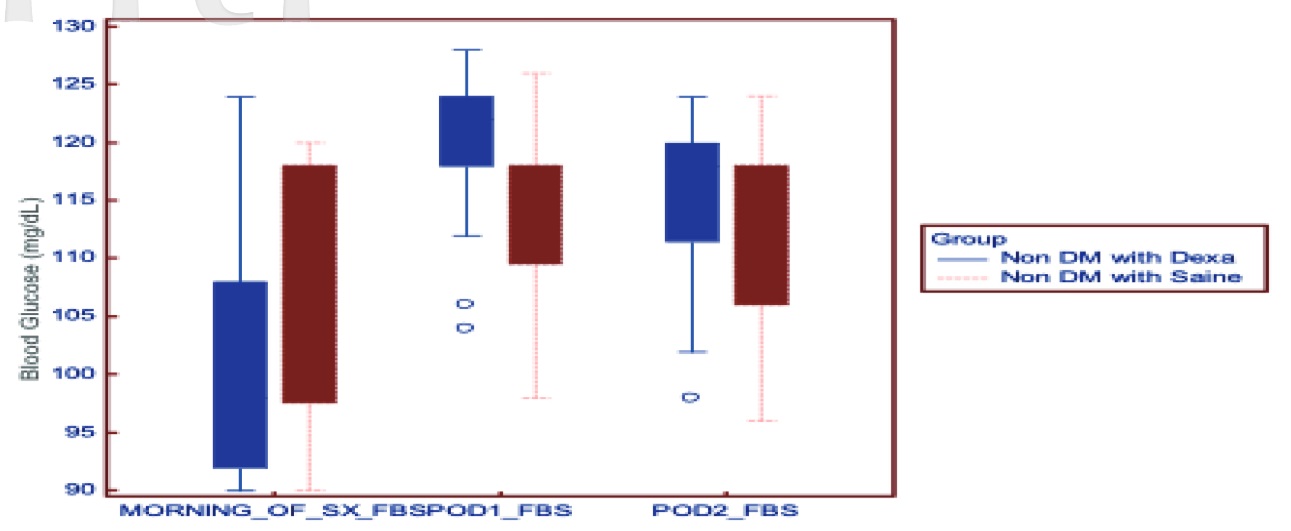
Download full-size image
Abbreviation: DM, diabetes mellitus.
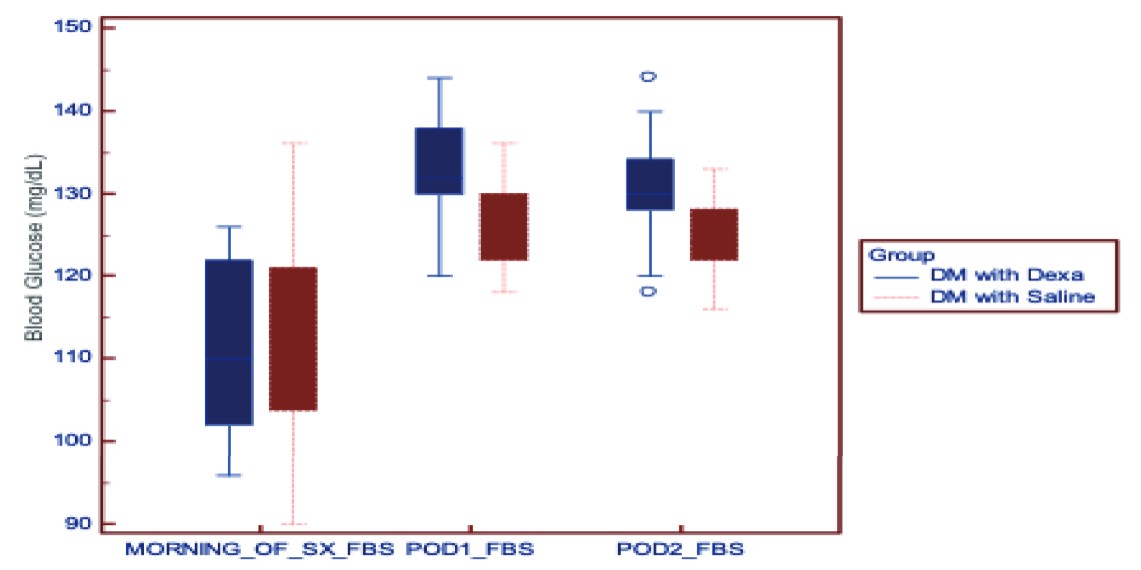
Download full-size image
Abbreviation: DM, diabetes mellitus.
Rise in Blood Sugar
Diabetics
The rise in blood glucose at 2 hours and 8 hours after dexamethasone in the dexamethasone group was 36.52 ± 9.51 [33.14, 39.89] mg/dL and 43.55 ± 9.72 [40.10, 46.99] mg/dL, respectively. Patients who received saline in the diabetic group had a rise of 16.76 ± 11.47 [12.69, 20.83] mg/dL after 2 hours and 22.09 ± 11.70 [17.94, 26.24] mg/dL after 8 hours.
The rise in blood glucose after 2 hours and 8 hours of dexamethasone was 35.18 ± 10.64 [31.41, 38.96] mg/dL and 41.64 ± 10.69 [37.85, 45.43] mg/dL, respectively. In the saline group, the rise in blood glucose 2 hours and 8 hours was 19.48 ± 9.41 [16.15, 22.82] mg/dL and 24.79 ± 9.70 [21.35, 28.23] mg/dL, respectively.
PONV
In the nondiabetics, 3 patients in the dexamethasone group and 8 patients in the saline group had PONV (
Discussion
Dexamethasone has been widely used as a prophylaxis for PONV for several years. A dose of 4–8 mg in adults at induction of anesthesia is recommended for PONV prophylaxis rather than at the end of the surgical procedure. 11 Dexamethasone takes up to 1–2 hours to act after its administration as it has to modify gene transcription. Therefore, it is ideally given after induction but before the start of surgery. 3,13 It has also been shown to enhance the quality of recovery and decrease the length of hospital stay. 14,15 Although there have been concerns regarding increased incidence of infection and bleeding in the postoperative period, several studies have allayed these concerns. 9 Other than its antiemetic action, it also has analgesic and anti-inflammatory actions during the perioperative period. 16 Different doses of dexamethasone and their effect on postoperative hyperglycemia have been studied. Low et al. 17 proved that dexamethasone dose of 8–10 mg is associated with a significantly greater increase in blood glucose compared with a 4 mg dose. Tien et al. 18 also noted hyperglycemic response to a single dose of 8 mg dexamethasone.
Surgery induces a stress response in any patient, and it can lead to various responses in the body including neuroendocrine and metabolic responses. 19,20 Stress hyperglycemia can occur as a part of this response, although it is transient. 21,22 The stress hormones released cause variations in carbohydrate metabolism, leading to insulin resistance in the liver and the skeletal muscles, which in turn causes hyperglycemia. This can occur in diabetics as well as nondiabetics, but stress hyperglycemia is related to long surgical duration as well as high ASA grading. 23 We have included patients undergoing laparoscopic surgeries (Laparoscopic cholecystectomy, hernia repair, appendicectomy, hysterectomy), and the mean duration of surgery was more than 2 hours in all the groups.
Studies have been done considering the hyperglycemic response to stress and the concomitant use of steroids in the intraoperative period, and hyperglycemic response was noted in both diabetics and nondiabetics. 24,25 Optimal glycemic levels could be different in patients with diabetes compared to nondiabetics 26 in the intraoperative period.
The PADDAG trial considered a single dose of either 4 mg or 8 mg dexamethasone and concluded that there was not a significant influence on the maximum blood glucose concentration in patients who received dexamethasone in both diabetics with good glycemic control and in nondiabetics.
27
We noted that there was a significant rise in blood sugar values in patients who received 8 mg dexamethasone compared to the saline group in nondiabetics, like in the diabetic group in our study. An intraoperative blood glucose concentration of more than 180 mg/dL can be considered as hyperglycemia.
28,29
None of our patients had blood glucose values more than 180 mg/dL after 2 hours and 8 hours of dexamethasone administration. Standard treatment care in our institution for perioperative hyperglycemia (blood glucose level more than 180 mg/dL) is to start variable rate insulin infusion, but none of the patients had this value. The FBS values on days 1 and 2 were also below 180 mg/dL in patients who received dexamethasone in both diabetics and nondiabetics. Although there was a significant increase in blood glucose concentration after receiving dexamethasone in both groups, there was no hyperglycemia
in nondiabetics and patients with well-controlled diabetes.
31-33
PONV can be very distressing to a patient in the recovery period, and it can hamper the quality of recovery. The dexamethasone group had a lower incidence of PONV (7.5 %) in our study compared to the saline group (19.6 %). All the patients in our study had more than one risk factor for PONV, considering that the duration of surgery was more than 60 minutes, and all of them underwent laparoscopic surgery under general anesthesia with volatile anesthetic agents and opioids. Guidelines suggest the use of two antiemetics when there are 1 and 2 risk factors for PONV. 11 We administered ondansetron to all the patients. The incidence of PONV was higher in our study; this could be because PONV prophylaxis was not given according to the scoring for the purpose of the study. However, none of the patients had a PONV score of 3. Previous dosage studies have shown that to prevent PONV after gynecological procedures, a minimum dose of 2.5 mg of dexamethasone is required. 34 Prophylactic dose of 8mg dexamethasone can suppress cortisol levels up to twenty hours in postoperative period; therefore, it should be used cautiously in patients with uncontrolled diabetes and severe insulin resistance. 35
There are limitations to this study. We did not include poorly controlled diabetic patients in our study. Patients with poor glycemic control have a higher chance of end-organ damage compared to well-controlled diabetics, and therefore, they are at risk of the deleterious effects of postoperative hyperglycemia. Since the safety profile in uncontrolled diabetes was not clear, we excluded such patients. Trials with higher sample sizes and monitoring the outcome of the patient for a longer duration will help us understand the effect of dexamethasone in surgical patients. 27
Conclusion
Dexamethasone can be considered as a prophylaxis for PONV in well-controlled diabetics and nondiabetics despite the increase in blood glucose levels, as a hyperglycemic response to a single dose was not observed in our study.
References
| 1 |
Jin Z, Gan TJ, Bergese SD.
Prevention and treatment of postoperative nausea and vomiting (PONV): a review of current recommendations and emerging therapies.
Ther Clin Risk Manag. 2020;16:1305-1317.
|
| 2 |
Jamtsho P, Dorjey Y, Dorji N, et al.
Factors associated with postoperative nausea and vomiting after laparoscopic cholecystectomy at the National Referral Hospital, Bhutan: a cross-sectional study.
BMC Anesthesiol. 2024;24(1):248.
|
| 3 |
Myles PS, Corcoran T.
Benefits and risks of dexamethasone in noncardiac surgery.
Anesthesiology. 2021;135(5):895-903.
|
| 4 |
Sapolsky RM, Romero LM, Munck AU.
How do glucocorticoids influence stress responses?
Endocr Rev. 2000;21(1):55-89.
|
| 5 |
Rhen T, Cidlowski JA.
Antiinflammatory action of glucocorticoids--new mechanisms for old drugs.
N Engl J Med. 2005;353(16):1711-1723.
|
| 6 |
DREAMS Trial Collaborators and West Midlands Research Collaborative.
Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial).
BMJ. 2017;357:j1455.
|
| 7 |
O’Connell RS, Clinger BN, Donahue EE, Celi FS, Golladay GJ.
Dexamethasone and postoperative hyperglycemia in diabetics undergoing elective hip or knee arthroplasty: a case control study in 238 patients.
Patient Saf Surg. 2018;12:30.
|
| 8 |
Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR.
Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total hip and knee arthroplasty.
J Arthroplasty. 2015;30(3):439-443.
|
| 9 |
Dobravc Verbič M, Gruban J, Kerec Kos M.
Incidence and control of steroid-induced hyperglycaemia in hospitalised patients at a tertiary care centre for lung diseases.
Pharmacol Rep. 2021;73(3):796-805.
|
| 10 |
Ali Z, Shah MA, Mir SA, Hassan N, Masoodi SR.
Effects of single dose of Dexamethasone on perioperative blood glucose levels in patients undergoing surgery for supratentorial tumors - An observational study.
Anesth Essays Res. 2020;14(1):56-61.
|
| 11 |
Gan TJ, Belani KG, Bergese S, et al.
Fourth consensus guidelines for the management of postoperative nausea and vomiting.
Anesth Analg. 2020;131(2):411-448.
|
| 12 |
Peter V, Shenoy U, Rukkiyabeevi B.
Effect of a single intraoperative dose of dexamethasone on glycaemic profile in postoperative patients - A double-blind randomised controlled study.
Indian J Anaesth. 2022;66(11):789-795.
|
| 13 |
Gan TJ, Diemunsch P, Habib AS, et al.
Consensus guidelines for the management of postoperative nausea and vomiting.
Anesth Analg. 2014;118(1):85-113.
|
| 14 |
Kleif J, Kirkegaard A, Vilandt J, Gögenur I.
Randomized clinical trial of preoperative dexamethasone on postoperative nausea and vomiting after laparoscopy for suspected appendicitis.
Br J Surg. 2017;104(4):384-392.
|
| 15 |
Backes JR, Bentley JC, Politi JR, Chambers BT.
Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial.
J Arthroplasty. 2013;28(8 Suppl):11-17.
|
| 16 |
De Oliveira GS, Almeida MD, Benzon HT, McCarthy RJ.
Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials.
Anesthesiology. 2011;115(3):575-588.
|
| 17 |
Low Y, White WD, Habib AS.
Postoperative hyperglycemia after 4- vs 8-10-mg dexamethasone for postoperative nausea and vomiting prophylaxis in patients with type II diabetes mellitus: a retrospective database analysis.
J Clin Anesth. 2015;27(7):589-594.
|
| 18 |
Tien M, Gan TJ, Dhakal I, et al.
The effect of anti-emetic doses of dexamethasone on postoperative blood glucose levels in non-diabetic and diabetic patients: a prospective randomised controlled study.
Anaesthesia. 2016;71(9):1037-1043.
|
| 19 |
Ivascu R, Torsin LI, Hostiuc L, Nitipir C, Corneci D, Dutu M.
The surgical stress response and anesthesia: a narrative review.
J Clin Med. 2024;13(10):3017.
|
| 20 |
Cusack B, Buggy DJ.
Anaesthesia, analgesia, and the surgical stress response.
BJA Educ. 2020;20(9):321-328.
|
| 21 |
Moghissi ES, Korytkowski MT, DiNardo M, et al.
American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.
Diabetes Care. 2009;32(6):1119-1131.
|
| 22 |
Davis G, Fayfman M, Reyes-Umpierrez D, et al.
Stress hyperglycemia in general surgery: why should we care?
J Diabetes Complications. 2018;32(3):305-309.
|
| 23 |
Sermkasemsin V, Rungreungvanich M, Apinyachon W, Sangasilpa I, Srichot W, Pisitsak C.
Incidence and risk factors of intraoperative hyperglycemia in non-diabetic patients: a prospective observational study.
BMC Anesthesiol. 2022;22(1):287.
|
| 24 |
Abdelmalak BB, Bonilla AM, Yang D, et al.
The hyperglycemic response to major noncardiac surgery and the added effect of steroid administration in patients with and without diabetes.
Anesth Analg. 2013;116(5):1116-1122.
|
| 25 |
Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V.
Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery.
Br J Anaesth. 2006;97(2):164-170.
|
| 26 |
Palermo NE, Gianchandani RY, McDonnell ME, Alexanian SM.
Stress hyperglycemia during surgery and anesthesia: pathogenesis and clinical implications.
Curr Diab Rep. 2016;16(3):33.
|
| 27 |
Corcoran TB, O’Loughlin E, Chan MTV, Ho KM.
Perioperative ADministration of Dexamethasone and blood glucose concentrations in patients undergoing elective non-cardiac surgery—the randomised controlled PADDAG trial.
Eur J Anaesthesiol. 2021;38(9):932-942.
|
| 28 |
Rajan N, Duggan EW, Abdelmalak BB, et al.
Society for Ambulatory Anesthesia Updated Consensus Statement on perioperative blood glucose management in adult patients with diabetes mellitus undergoing ambulatory surgery.
Anesth Analg. 2024;139(3):459-477.
|
| 29 |
Duggan EW, Carlson K, Umpierrez GE.
Perioperative hyperglycemia management: an update.
Anesthesiology. 2017;126(3):547-560.
|
| 30 |
Murphy GS, Szokol JW, Avram MJ, et al.
The effect of single low-dose dexamethasone on blood glucose concentrations in the perioperative period: a randomized, placebo-controlled investigation in gynecologic surgical patients.
Anesth Analg. 2014;118(6):1204-1212.
|
| 31 |
Yurkonis AV, Tollinche L, Alter J, et al.
Standardizing the dosage and timing of dexamethasone for postoperative nausea and vomiting prophylaxis at a Safety-Net Hospital System.
Jt Comm J Qual Patient Saf. 2024;50(8):601-605.
|
| 32 |
Asehnoune K, Le Moal C, Lebuffe G, et al.
Effect of dexamethasone on complications or all cause mortality after major non-cardiac surgery: multicentre, double blind, randomised controlled trial.
BMJ. 2021;373:n1162.
|
| 33 |
Corcoran TB, Myles PS, Forbes AB, et al.
Dexamethasone and surgical-site infection.
N Engl J Med. 2021;
384(18):1731-1741. |
| 34 |
Ho CM, Wu HL, Ho ST, Wang JJ.
Dexamethasone prevents postoperative nausea and vomiting: benefit versus risk.
Acta Anaesthesiol Taiwan. 2011;49(3):100-104.
|
| 35 |
Cowie BS, Allen KJ, Said SA, Inder WJ.
Anti-emetic doses of dexamethasone suppress cortisol response in laparoscopic cholecystectomy.
Anaesth Intensive Care. 2010;38(4):667-670.
|