Abstract
Objective
To evaluate the analgesic effi cacy of dexamethasone (DXA) vs. dexmedetomidine (DXM) as an adjunct to ropivacaine in ultrasound-guided interscalene block (USG-ISB) for arthroscopic shoulder surgery.
Methods
In this randomized double-blinded controlled trial, 60 American Society of Anesthesiologists grade 1–2 patients, 18–65 years, scheduled for arthroscopic shoulder surgery were randomly allocated to either group 1 (USG-ISB given with 0.5% ropivacaine 20 mL with 2 mL of saline containing DXM 0.5 mcg/kg) or group 2 (same protocol but DXA 8 mg instead of DXM). ISB was performed with in-plane technique under USG. Following surgery under general anesthesia, the patients received intravenous patient-controlled analgesia with fentanyl. Data were collected at 2-hourly intervals up to 24 h after USGISB. The primary outcome was the duration of analgesia. Secondary outcomes consisted of pain ratings, total cumulative postoperative fentanyl consumption, patient satisfaction, and adverse effects.
Results
The groups were comparable regarding baseline demographic and clinical characteristics including onset of sensory and motor block. The duration of postoperative analgesia for group 2 was signifi cantly longer than for group 1 (22.40 ± 2.16 vs. 19.30 ± 3.80 h; p < 0.001). Group 2 also required less total median number of boluses than group 1 (0 vs. 3; p < 0.001), less total fentanyl consumption (10.00 ± 24.20 vs. 40.33 ± 38.70 mcg; p < 0.001), less pain scores, and greater satisfaction (99.30 ± 2.53 vs. 93.30 ± 11.50; p = 0.007). Adverse effects were few and comparable in both groups.
Conclusion
Greater postoperative analgesia and opioid sparing effect was observed in patients receiving 8 mg DXA as adjunct for USG-ISB.
Keywords
analgesic, adjunct, dexmedetomidine, dexamethasone, ultrasound-guided interscalene block, arthroscopic shoulder surgery
Introduction
Shoulder surgeries are associated with substantial pain, which often persists beyond the usual duration of analgesia produced by nerve blocks and therefore may be helped by regional anesthetic techniques.1 Interscalene block (ISB) is an established anesthetic-analgesic technique used during surgery of the shoulder and upper limb.2 Ultrasound-guided ISB (USG-ISB) is a technological advancement. It increases duration, improves block success, block onset, and block quality and reduce mean effective anesthetic volume and mean effective anesthetic concentration of local anesthetic.3,4
The addition of various adjuvants like opioids, α-2 agonists, ketamine, neostigmine, magnesium sulphate, adenosine, and steroids to local anesthetics hasten the onset and prolong the duration of analgesia.5
Dexamethasone (DXA) and dexmedetomidine (DXM) have been independently shown to prolong the duration of analgesia when added with ropivacaine perineurally for brachial plexus block.6,7 However, there are only a few studies directly comparing the efficacy of DXA and DXM with ropivacaine or bupivacaine for different types of brachial plexus blocks, with inconsistent findings.8-11
In view of these few studies with contradictory findings, the current study was designed with the aim to evaluate the efficacy of perineural DXA vs. perineural DXM as an adjunct to ropivacaine in increasing the duration of postoperative analgesia, evaluate the difference in postoperative pain scores, total postoperative analgesic consumption in 24 h, and patient satisfaction. The null hypothesis was that there would be no significant difference between the two groups with regard to duration of postoperative analgesia and related outcomes.
Methods
The trial was registered with the Clinical Trials Registry-India (CTRI; trial registration number CTRI/2015/08/006124, dated August 20, 2015). Patients were enrolled following CTRI registration from September 2015 till August 2016. Fig. 1 shows the CONSORT flow diagram.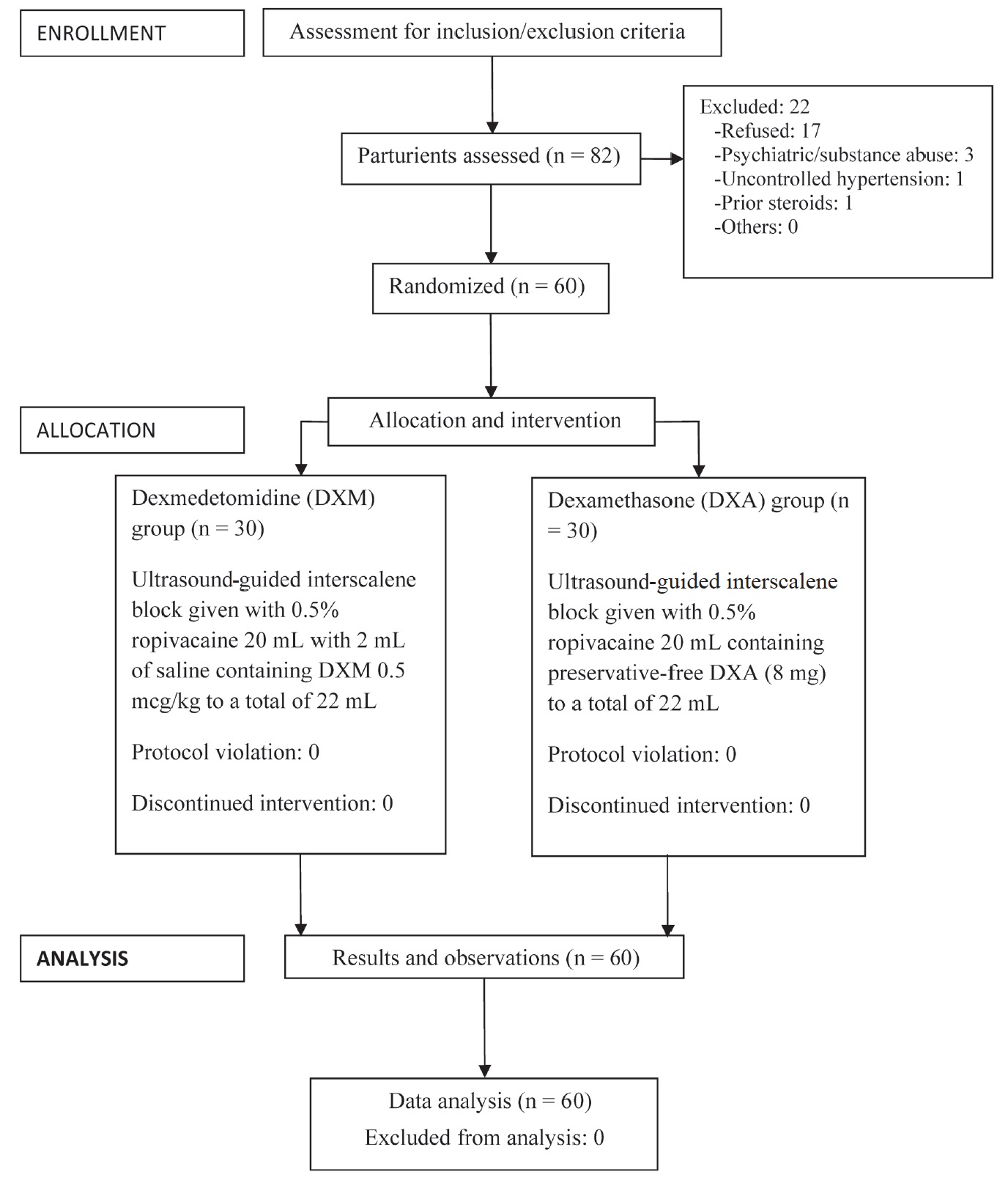
Download full-size image
After obtaining approval from the Institutional Ethics Committee, 60 patients belonging to American Society of Anesthesiologists (ASA) grade 1 or 2, aged 18-65 years, body mass index (BMI) 18-30 kg/m2, admitted in hospital for arthroscopic shoulder surgery (rotator cuff repair, and Bankart procedure for recurrent shoulder dislocation) were included in the study after obtaining written informed consent.
Patients refusing to give informed consent, undergoing revision surgery, with history of relevant drug allergy, with history of psychiatric illness, substance abuse, severe cardiovascular, respiratory, metabolic or neurological disease, coagulopathy, contralateral phrenic nerve dysfunction, infection at injection site, receiving α-2 adrenergic agonists or on steroids were excluded from the study.
Using computer generated random number table, the patients were randomly allocated to one of the following groups using 60 coded opaque sealed envelopes:
(1) Group 1 (n = 30): USG-ISB given with 0.5% ropivacaine 20 mL with 2 mL of saline containing DXM 0.5 mcg/kg to a total of 22 mL.
(2) Group 2 (n = 30): USG-ISB given with 0.5% ropivacaine 20 mL containing preservative-free DXA (8 mg) to a total of 22 mL.
The study drug was prepared in similar-looking 20 mL syringes by an anesthesiologist not involved in the study. The patient and the investigator performing and assessing the ISB were blinded to the study drug.
Preoperatively, a complete preanesthetic evaluation and investigations were performed for all the patients. They were explained about linear visual analog scale (VAS) for pain (0 = no pain, 100 = worst imaginable pain) and use of patient controlled analgesia (PCA) in their own vernacular language. Patients were kept fasting for 6 h minimum and were premedicated with oral ranitidine 150 mg and oral alprazolam 0.25 mg on the night before surgery and at 6 am on the morning of surgery.
Description of Procedure
The block was performed in the operating room (OR). Oxygen supplementation was provided by nasal prongs in all the patients. Patients were connected to a multichannel monitor (GE Healthcare, Helsinki, Finland) and monitored for electrocardiogram (ECG) and heart rate (HR), non-invasive arterial blood pressure (NIBP) and oxygen saturation (SpO2). An intravenous infusion of normal saline at rate of 2 mL/kg/h was started after inserting 18 G peripheral intravenous cannula. The procedure was performed in head up position with head rotated towards the non-operative side. After ensuring full aseptic conditions and draping the area, local infiltration of skin was performed with 2% lidocaine. A high-frequency (5-10 MHz) ultrasound probe (Sonosite Inc., Bothell, WA, USA) was placed transversely at the level of cricoid cartilage. The transducer was moved laterally towards the operative side to identify the carotid artery and internal jugular vein. Further lateral, anterior scalene and medial scalene muscle was identified. The roots of the brachial plexus were seen between the two muscles as three hyperechoic vesicles lying in close proximity with each other. The block was performed with inplane technique with 22 G, 40 mm Sonoplex stim cannula (PAJUNK GmbH, Geisingen, Germany) and the drug was deposited under ultrasound guidance as per the group allocation. After injection of the study drug, patients were evaluated at 2-min intervals for 10 min for development of sensory and motor block. Sensory block was assessed by loss of sensation to pinprick over the deltoid muscle. It was assessed using a 3-point scale to pinprick with a toothpick (0 = pinprick to shoulder, normal sensation; 1 = sharp to pinprick; 2 = pinprick felt but not sharp; 3 = no sensation, pinprick not felt). Time to achieve adequate sensory block was noted. Motor block was assessed by failure to abduct the shoulder, the so-called “deltoid sign” (0 = normal abduction; 1 = decreased movement, moves shoulder but not normal; 2 = unable to abduct shoulder). After assessing the sensory and motor blockade at regular intervals, all the patients were administered general anaesthesia.
Anesthesia in all patients was induced with injection of fentanyl 2 mcg/kg, propofol 2 mg/kg and vecuronium 0.1 mg/kg to facilitate endotracheal intubation insertion. Anesthesia was maintained with nitrous oxide/oxygen (N2O/O2) in a ratio of 60:40 with isoflurane (inspired concentration of 0.5-2.0%). After ensuring bilateral air entry, surgical procedure was allowed to proceed. There was provision to supplement fentanyl intra-operatively in the dose of 1 mcg/kg if there was 20% increase from the baseline parameters. After the induction of anaesthesia, end-tidal carbon dioxide (EtCO2) was also monitored in addition to the above parameters.
Fifteen min before completion of surgery, an injection of ondansetron 0.1 mg/kg was given in all patients under study. At the end of the procedure, the residual neuromuscular block was reversed with injection neostigmine 50 mcg/kg intravenous (IV) injection and injection glycopyrrolate 10 mcg/kg IV. Subsequently the patient’s trachea was extubated and the patients were shifted to post anaesthesia care unit.
The patients were connected to IV-PCA (Master PCA, Fresenius Kabi, Bad Homburg, Germany) providing fentanyl boluses as analgesic. The pump was set to deliver patient-controlled boluses of 10 mcg of fentanyl, with lock-out interval of 6 min, maximum 4-h dose of fentanyl being 400 mcg. The patients were discharged 48 h after admission.
Data Collection
The data were collected for 24 h after USG-ISB. Demographic (age, gender, and comorbidities) and morphometric (height and weight) characteristics of participating patients were recorded. After induction, all the patients were monitored for hemodynamic variables at regular intervals. Intra operative HR, systolic blood pressure, diastolic blood pressure and respiratory rate were measured at 0, 5, 10, 15, 20, 40, 60, and 80 min, respectively. After the surgery, the patients were kept in the post anaesthetic care unit (PACU). Postoperative HR, systolic blood pressure, diastolic blood pressure, and respiratory rate were measured at 2-hourly intervals till 24 h. In the PACU, pain was assessed using VAS scoring system. All the observations (age, sex, and vital parameters) including VAS for pain, occurrence of adverse effects using a checklist, total analgesic requirements, and total antiemetic requirements were recorded.
Primary Outcome
Duration of analgesia (defined as time from onset of adequate sensory block to the time of patient self-administering the first bolus of supplemental analgesic medication).
Secondary Outcomes
Pain score, which was evaluated using VAS at 2-h increments starting after the nerve block (“0 hour”) over a total of 24 h; patient satisfaction on a 0-100 VAS; total cumulative post-operative analgesic consumption (fentanyl mcg), and adverse effects were noted. The sedation in two groups was assessed at 6 h after the nerve block using Ramsay sedation scale (RSS).12 RSS scores on sedation are marked on a 6-point scale on increasing levels of sedation, 1 being “patient anxious or agitated or both,” and 6 being “no response to light, glabellar tap, or loud auditory stimulus.” A score of 3 or more is considered as indicative of sedation.
Sample Size Calculation
Because almost all patients require analgesics after shoulder procedures, sample size estimation assumed no censoring of block durations. Based on the data of a previous study that used 8 mg DXA as adjuvant to 0.5% ropivacaine for ISB,6 and considering 25% change in duration of analgesia with adjuvant DXM as clinically significant, a sample size of 26 subjects in each group would have 90% power to detect a significant difference, assuming equal variance using a 2-group t test with a 0.05 two-sided significance level. After allowing for 10% noncompletion rate, the study was designed to enrol 60 total subjects (30 per group).
Statistical Analysis
The statistical analysis was carried out using Statistical Package for Social Sciences version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were tested for normal distribution using Kolmogorov-Smirnov test. To test for significance of difference between the two groups on single-measure data (patient satisfaction score at the end of 24 h study period, and total postoperative fentanyl consumption till 24 h postoperatively), between-group comparisons was performed using a Student’s t test for normally distributed continuous variables, Mann-Whitney U test for non-normally distributed continuous variables, chi-square test for binary variables, and Wilcoxon test for ordered categorical variables. For repeated-measures data assessment over time, including pain scores at rest and with activity, postoperative sensory and motor scores, HR, and blood pressure, two-way repeated- measures analysis of variance (ANOVA) with post-hoc Scheffe’s test was used for analysis. Significance level was set at 0.05.
Results
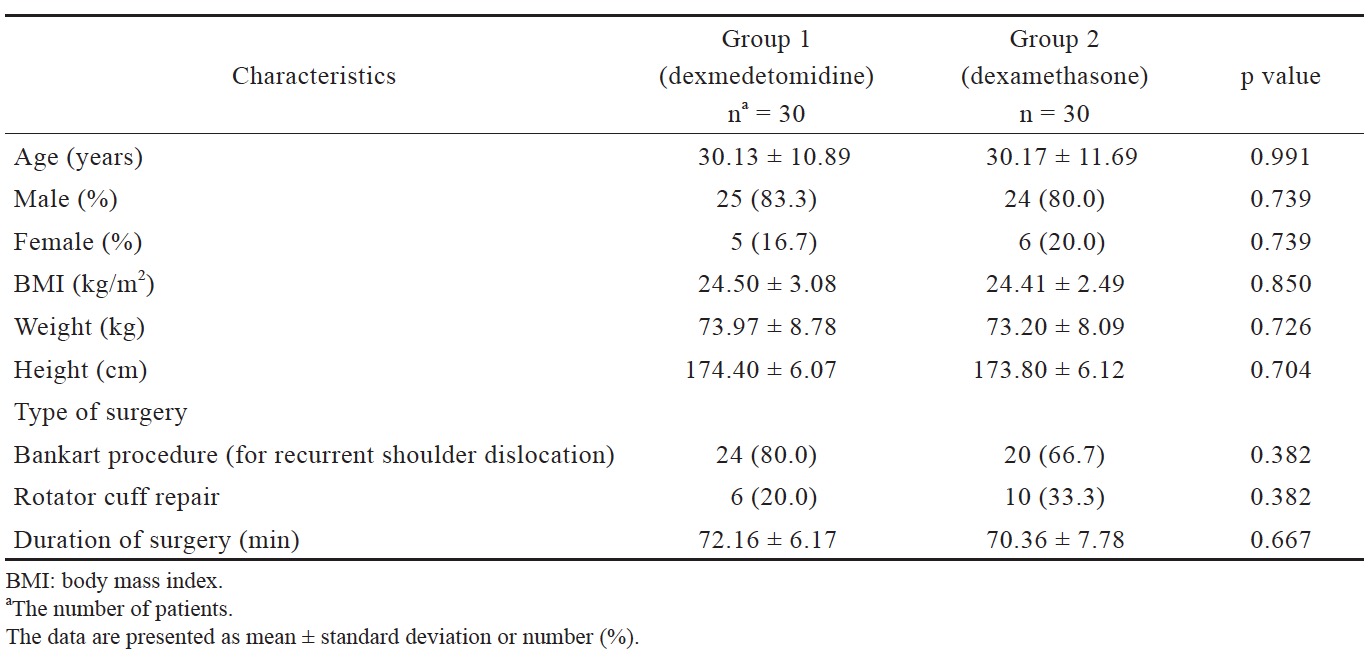
There was no statistically significant difference in the two groups with respect to age, sex, height, weight, BMI, type, and duration of surgery (Table 1).
Download full-size image
There was no significant difference between the groups in relation to preoperative investigations and vital parameters. Sensory block onset and motor block onset was also not significantly different. Sensory block onset for group 1 was 2.33 ± 0.47 min and for group 2 was 2.53 ± 0.51 min. Motor block onset for group 1 was 5.06 ± 1.55 min and for group 2 was 5.73 ± 0.98 min.
Differences in intra operative HR, systolic blood pressure, diastolic blood pressure, and respiratory rate between the groups were not significant. Differences in postoperative HR, systolic blood pressure, diastolic blood pressure, and respiratory rate between the groups were also not significant.
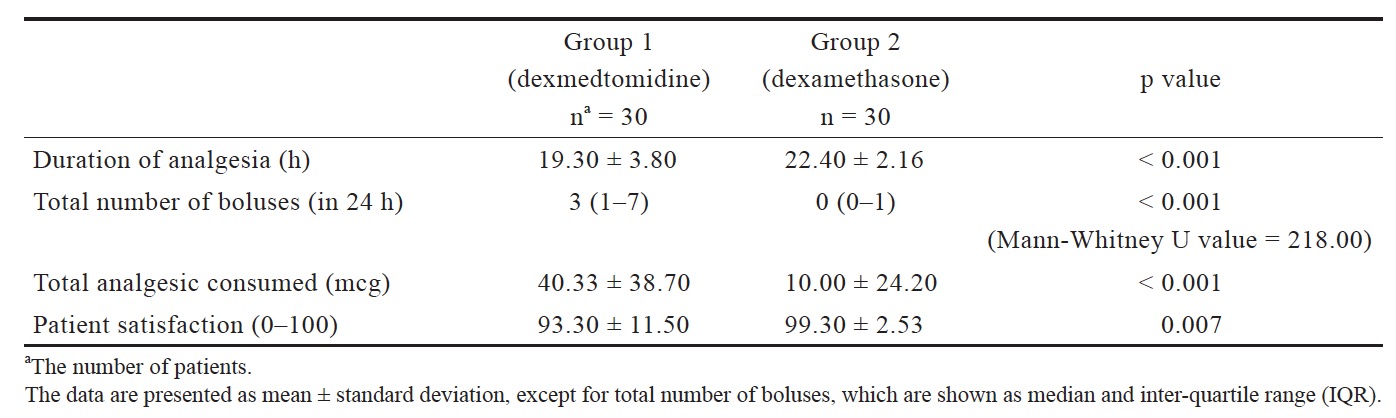
In regard to the primary outcome measure, the duration of analgesia for group 2 was 22.40 ± 2.16 h and for group 1 it was 19.30 ± 3.80 h. The difference was statistically significant (p < 0.001), and it showed that group 2 had significantly longer duration of analgesia compared to group 1 (Table 2).
Download full-size image
Total number of boluses used in 24 h was significantly more in group 1 (median = 3, interquartile range [IQR] = 1-7) as compared to group 2 (median = 0, IQR = 0-1; p < 0.001) (Table 2).
Total analgesic consumption was 40.33 ± 38.70 mcg for group 1 and 10.00 ± 24.20 mcg for group 2, with significant difference between the two groups (p < 0.001; Table 2).
Two-way ANOVA showed that the VAS pain scores at rest and movement decreased significantly with time in both the groups. However, there was significant intergroup difference for both pain scores at rest (F = 21.7, p < 0.001) and on movement (F=25.5, p < 0.001). On application of post-hoc Scheffe’s test, group 2 scored significantly less than group 1 on pain scores at rest and on movement 16 h onwards postoperatively till the data collection period. This was confirmed by the group × time interaction term in twoway ANOVA, which was significant for both pain at rest (F=9.74, p < 0.001) and pain on movement (F=20.89, p < 0.001).
Patient satisfaction was significantly different between the two groups. It was 93.30 ± 11.50 for group 1 and 99.30 ± 2.53 for group 2 (p = 0.007; Table 2).
No episodes of dizziness, vertigo, hypotension, hypoxemia, decreased respiratory rate, or bradycardia was seen within 24 h postoperatively. More patients in group 1 were sedated as compared to group 2 (5 patients in group 1 scored 3 or more on RSS compared to none in group 2) though the difference was not statistically significant (p = 0.522). Two patients scored 4 (both in group 1), and none scored 5 or 6 (deep sedation). Horner’s syndrome and hoarseness of voice were seen among both the groups. Horner’s syndrome was seen in one patient in group 1 (3.33%) and in two patients in group 2 (6.66%). Hoarseness of voice was seen in two patients in group 1 (6.66%) and in one patient in group 2 (3.33%). There were no episodes of nausea or vomiting or any other side effects.
Discussion
Two main findings emerged in this randomized double blind study comparing 0.5 mcg/kg DXM with 8 mg DXA, both administered perineurally as an adjunct to 0.5% ropivacaine in USG-ISB for arthroscopic shoulder surgery. First, DXA as an adjunct resulted in an average 3 h of additional duration of analgesia compared to adjunct DXM. This was corroborated by the two-way ANOVA showing significant group × time interaction in pain scores, with Scheffe’s post-hoc test documenting significant inter-group differences in pain scores at rest and on movement emerging after 16 h of block. We cannot comment if the duration of analgesia may actually be even longer in the DXA group, since the data collection in our study was truncated at 24 h.
The second important finding is that the on-demand opioid consumption was significantly less in the DXA group, which consumed an average 30 mcg less fentanyl by IV-PCA than the DXM group. This difference is not only statistically significant (p = 0.007) but is potentially clinically meaningful.
In addition to the longer duration of analgesia and less requirement of additional opioid analgesic, a reassuring finding was that the adverse effects were quite low and not serious in both the groups. DXM was somewhat more sedating than DXA, but no one in either group was grossly sedated. Gurajala et al.13 hypothesized that perineural DXM reaches systemic circulation in sufficient concentrations to cause sedation. Other adverse effects such as hoarseness of voice and Horner’s syndrome were observed in 1-2 patients (3-6%) in either group. These figures were actually lower than those of Desmet et al.14 who reported 20.5% of Horner’s syndrome with ropivacaine and 23.5% with ropivacaine plus DXA. The lower incidence of these in our patients could be due to the lower volume of local anesthetic and adjuvants used in our study.
In our study, 8 mg of DXA was used as perineural adjunct to the nerve block. Desmet et al.14 used 0.5% of 30 mL ropivacaine with 10 mg DXA and duration of analgesia was 23 h. Cummings et al.6 used 0.5% of 30 mL ropivacaine with 8 mg DXA and the median duration of analgesia was 22.4 h. The previously cited studies directly comparing DXA with DXM as perineural adjuvants for brachial plexus blocks all used DXA in the doses of 8-10 mg.8-11 Although later studies often used lower doses of DXA (e.g., Kawanishi et al.15 used 0.75% of 20 mL ropivacaine with 4 mg DXA) there is sufficient precedence of using DXA at 8 mg dose with no evidence of adverse effects at least during the observation periods of the studies.
The route of DXA administration has been debated in the past and it was reported that there was no difference in the analgesic effect between perineural and systemic administration.16 DXA has been specifically studied as an adjunct to epidural local anaesthetics. 17 The neurological risk with its use appears to be small.18
The precise mechanism of action of DXA added to local anesthetics is not known but studies have described direct effect of glucocorticoids on nerve conduction and induction of perineural vasoconstriction with absorption of local anesthetics.19 On the other hand, DXM produces analgesia by inhibition of substance P release in nociceptive pathway and activation of α-2 adrenoceptors in the locus coeruleus.20 When used in nerve blocks, it produces analgesic effect by enhancement of hyperpolarisation of activated cation channel.21
Several studies and a meta-analysis have shown that DXM increases the duration of analgesia by 6-8 h when added to local anesthetic in a nerve block.22-25 It is also known that DXA does the same as an adjunct to regional analgesia, based on many individual studies and a meta-analysis of 29 studies.6,26
Thus, while both DXA and DXM have been shown to prolong analgesia when used as an adjunct with ropivacaine in individual studies, only a few direct head-to-head comparisons are available, and with inconclusive or even contradictory results.8-11 Kumar8 compared 0.25% bupivacaine plus 8 mg DXA with 0.25% bupivacaine plus 50 mcg DXM as an adjuvant for interscalene brachial plexus block. They found that duration of motor block and sensory block was significantly prolonged in DXA group compared to DXM group. However, there were no major differences in opioid and back up analgesic requirements in the two groups.8 In contrast, Verma and Ranjan, who compared 0.5% ropivacaine plus 8 mg DXA with 0.5% ropivacaine plus 50 mcg DXM for supraclavicular brachial plexus block, found that has DXM produced earlier onset with longer duration of analgesic action in comparison to DXA.9 Another recent study too found 50 mcg DXM as adjuvant to a combination of 2% lignocaine-adrenaline and 0.5% bupivacaine for supraclavicular block produced earlier onset and prolonged duration of motor (but not sensory) block compared to 8 mg DXA.10 Finally, Lee et al. did not find any significant difference between DXA and DXM even when they compared 10 mg of DXA with 100 mcg DXM as adjuvant with 0.5% ropivacaine for USG axillary brachial plexus block with nerve stimulation.11
The inconsistency in the findings of the previous studies might be because of several methodological factors such as use of different local anesthetics, different techniques of brachial plexus block, and use of additional nerve stimulation. In this study, we directly compared these two agents for USG-IGB with a robust methodology.
Most patients in both the groups in our study were satisfied with the pain management experience as evidenced by the satisfaction scores, though almost everyone in the DXA group was satisfied vis-à-vis 9 out of 10 in the DXM group, the difference being statistically significant. Patient demographics and hemodynamic parameters were also comparable between the groups.
This study had a few limitations. First, it included only ASA physical status 1 and 2 patients; hence the results of the study should not be generalized to patients of higher grades of ASA physical status. Second, the serum levels of ropivacaine and DXM were not measured. Third, the length of hospital stay and evaluation of long term benefits of pain relief would have been more valuable and would have added more strength to the study. Fourth, sensory block was not assessed using repeated neurological examination due to difficulty in assessing sensory block after surgery; hence the first analgesic bolus use was used as a proxy measure for the end point of sensory block. Finally, our patients used fentanyl through IVPCA following the nerve block if needed, rather than non-opioid analgesics first. This was done to clearly demarcate the duration of analgesia produced by the local anesthetic-adjunct combination as perceived subjectively by the patients, thus making it clinically relevant. Generalizability of the findings of this study should be limited to these considerations. However, we believe that none of these limitations can invalidate the major findings of the study within the limits of their applicability and interpretability.
In conclusion, this study shows that greater postoperative analgesia was observed in patients receiving perineural 8 mg DXA with 20 mL of 0.5% ropivacaine using USG-IGB as compared to 0.5 mcg/kg of DXM with 20 mL of 0.5% ropivacaine. DXA when used as an adjunct had more opioid sparing effect as compared to DXM when used in conjunction with 0.5% ropivacaine in USG-IGB in patients undergoing arthroscopic shoulder surgery.
Conflicts of Interest
None.
References
| 1 |
Liu SS, Strodtbeck WM, Richman JM, Wu CL.
A comparison of regional versus general anaesthesia for ambulatory anaesthesia: a meta-analysis of randomized controlled trials.
Anesth Analg 2005;101:1634-1642.
|
| 2 | |
| 3 |
Kapral S, Greher M, Huber G, et al.
Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade.
Reg Anesth Pain Med 2008;33:253-258.
|
| 4 |
Soeding PE, Sha S, Royse CE, Marks P, Hoy G, Royse AG.
A randomized trial of ultrasound-guided brachial plexus anaesthesia in upper limb surgery.
Anaesth Intensive Care 2005;33:719-725.
|
| 5 |
Yilmaz-Rastoder E, Gold MS, Hough KA, Gebhart GF, Williams BA.
Effect of adjuvant drugs on the action of local anesthetics in isolated rat sciatic nerves.
Reg Anesth Pain Med 2012;37:403-409.
|
| 6 |
Cummings KC III, Napierkowski DE, Parra-Sanchez I, et al.
Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine.
Br J Anaesth 2011;107:446-453.
|
| 7 |
Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M.
Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study.
Br J Anaesth 2013;110:438-442.
|
| 8 |
Kumar AN.
Comparative study between 0.5% Bupivacaine with 8 M.G dexamethasone and 0.25% bupivacaine with 50μg dexmedetomidine as an adjuvant for interscalene brachial plexus block: prospective clinical study.
J Evol Med Dent Sci 2014;3:13111-13119.
|
| 9 |
Verma NK, Ranjan A.
A clinical comparison of dexmedetomidine and dexamethasone as adjuvant to ropivacaine in supraclavicular brachial plexus blocks for upper arm surgeries.
Int J Adv Res Biol Sci 2016;3:56-61.
|
| 10 |
Kaur M, Lakhani A, Hashia AM.
Comparative study between dexamethasone and dexmedetomidine in supraclavicular block.
Int J Adv Med 2018;5:57-61.
|
| 11 |
Lee MJ, Koo DJ, Choi YS, Lee KC, Kim HY.
Dexamethasone or dexmedetomidine as local anesthetic adjuvants for ultrasound-guided axillary brachial plexus blocks with nerve stimulation.
Korean J Pain 2016;29:29-33.
|
| 12 |
Ramsay MA, Savege TM, Simpson BR, Goodwin R.
Controlled sedation with alphaxalone-alphadolone.
BMJ 1974;2:656-659.
|
| 13 |
Gurajala I, Thipparampall AK, Durga P, Gopinath R.
Effect of perineural dexmedetomidine on the quality of supraclavicular brachial plexus block with 0.5% ropivacaine and its interaction with general anaesthesia.
Indian J Anaesth 2015;59:89-95.
|
| 14 |
Desmet M, Braems H, Reynvoet M, et al.
I. V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study.
Br J Anaesth 2013;111:445-452.
|
| 15 |
Kawanishi R, Yamamoto K, Tobetto Y, et al.
Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial.
Local Reg Anesth 2014;7:5-9.
|
| 16 |
Rahangdale R, Kendall MC, McCarthy RJ, et al.
The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study.
Anesth Analg 2014;118:1113-1119.
|
| 17 |
Khafagy HF, Refaat AI, El-Sabae HH, Youssif MA.
Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia.
J Anesth 2010;24:531-536.
|
| 18 |
Racz GB, Noe CL.
Pelvic spinal neuraxial procedures.
In: Raj P, Lou L, Serdar E, et al, eds. Interventional Pain Man-agement. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2008.
|
| 19 |
Wang PH, Tsai CL, Lee JS, Wu KC, Cheng KI, Jou IM.
Effects of topical corticosteroids on the sciatic nerve: an experimental study to adduce the safety in treating carpal tunnel syndrome.
J Hand Surg Eur 2011;36:236-243.
|
| 20 |
Gabriel JS, Gordin V.
Alpha 2 agonists in regional anesthesia and analgesia.
Curr Opin Anaesthesiol 2001;14:751-753.
|
| 21 |
Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R.
Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat.
Anesthesiology 2009;111:1111-1119.
|
| 22 |
Fritsch G, Danninger T, Allerberger K, et al.
Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial.
Reg Anesth Pain Med 2014;39:37-47.
|
| 23 |
Das A, Majumdar S, Haldar S, et al.
Effect of dexmedetomidine as adjuvant in ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded and randomized controlled study.
Saudi J Anaesth 2014;8(Suppl 1):S72-S77.
|
| 24 |
Bharti N, Sardana DK, Bala I.
The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: a randomized controlled trial.
Anesth Analg 2015;121:1655-1660.
|
| 25 |
Abdallah FW, Brull R.
Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis.
Br J Anaesth 2013;110:915-925.
|
| 26 |
Albrecht E, Kern C, Kirkham KR.
A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks.
Anaesthesia 2015;70:71-83.
|