Abstract
Objective
Epidural anesthesia for the parturient is often provided in a clinical context where rapid onset of segmental analgesia is important; however, little is published on the ideal local anesthetic to safely achieve this onset. To fi ll this gap in knowledge, we studied bupivacaine and lidocaine, two local anesthetics (LA) commonly used for labor epidural activation, either as a single drug or in combination to determine the onset of epidural analgesia.
Methods
In this double-blinded study, seventy-five patients were randomized into three groups (n = 25 each) for labor epidural activation: 10 mL of 0.25% bupivacaine, 10 mL of 1% lidocaine, or 5 mL of 0.25% bupivacaine plus 5 mL of 1% lidocaine. Patients were assessed for the fi rst 20 min after drug administration at 5-min intervals. Data collected included sensory level to pinprick, maternal blood pressure, vasopressor administration, and peak motor blockade.
Results
Data were analyzed on 71 of 75 patients. Time to loss of sensation to pinprick at the T10 dermatome in the bupivacaine group was signifi cantly longer than the lidocaine group (p = 0.006), but the time to loss of sensation to pinprick at the T10 dermatome did not signifi cantly differ in the bupivacaine plus lidocaine group when compared to both the bupivacaine (p = 0.114) as well as the lidocaine (p = 0.203) groups. Phenylephrine usage did not signifi cantly differ amongst the three groups (p = 0.062).
Conclusion
Lidocaine provides statistically signifi cant faster onset of epidural analgesia when compared to bupivacaine only. Combining the two LA did not signifi cantly affect onset.
Keywords
epidural analgesia, labor pain, local anesthetics, obstetrical analgesia
Introduction
Epidural anesthesia is an effective method for controlling pain during labor and delivery.1 It is often provided in a clinical context where rapid onset of segmental analgesia is extremely important. A typical scenario is the patient with rapidly progressing labor or the parturient with a distressed fetus where urgent cesarean delivery becomes necessary. The question about the best choice of local anesthetic to provide fast onset of epidural analgesia or anesthesia is clinically extremely important. Despite the clinical significance of this question, only a few studies have been published to quantify the speed of onset for commonly used local anesthetics (LA) or combination of LA when used to provide epidural analgesia.
Two common LA used to provide labor analgesia are bupivacaine and lidocaine. Although both have been safely administered for years, neither achieves the properties of an ideal local anesthetic needed for the initial bolus to “activate” the epidural. Bupivacaine has a slow onset of action when compared to lidocaine; 2 whereas, lidocaine has been shown to cause more maternal hypotension.3,4 Sinatra et al. found no significant difference in the time of onset or the extent of the sensory and motor block in parturients receiving bupivacaine or a combination of bupivacaine and lidocaine for labor analgesia.5 In patients requiring conversion of a labor epidural to a surgical block, it has been shown that there is no difference in time to achieve a T4 surgical level with a bolus of lidocaine, bupivacaine, or combination of the two.6 Seow et al. demonstrated no difference in the development of a sensory block with differing mixtures of lidocaine, bupivacaine, and lidocaine plus bupivacaine in non-pregnant patients undergoing lower abdominal surgery.7
Given the limited evidence for the usefulness of the bupivacaine and lidocaine mixture as it relates to labor epidural activation, we deigned a clinical study to quantify the speed-of-onset for bupivacaine, lidocaine and the combination of both LA. The primary aim of this study was to determine the time to achieve loss of sensation to pinprick at theT10 dermatome. Secondary aims included determining: (1) the peak motor blockade; (2) the largest change in mean arterial blood pressure; and (3) the need for vasopressor therapy to treat hypotension.
Methods
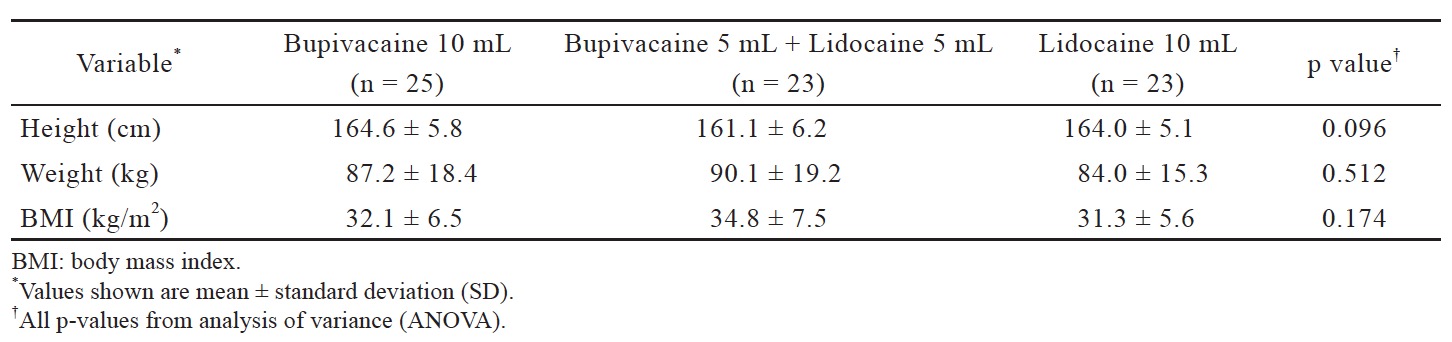
This study was approved on October 9, 2014 by the University of Alabama at Birmingham institutional review board (IRB) and registered at ClinicalTrials. gov (NCT03103100). All patients gave informed written consent to participate in the study. Any patient admitted to our labor and delivery unit for induction of labor and did not meet the exclusion criteria was eligible for the study. Exclusion criteria were: (1) age less than 19 years old; (2) allergy to the drug or drug class; (3) history of preexisting neuropathy; (4) history of back pain or back surgery prior to pregnancy; (5) history of chronic opioid use; (6) history of hypertension or hypertensive disorder of pregnancy; (7) history of congenital or acquired cardiac disease; (8) contraindication to epidural placement. After written consent was obtained, the patient was randomly assigned to one of three groups: (B) 10 mL of 0.25% bupivacaine; (L) 10 mL of 1% lidocaine; (BL) 5 mL of 0.25% bupivacaine plus 5 mL of 1% lidocaine. Drug concentrations were chosen based on the understanding that bupivacaine is four times as potent as lidocaine.2 Although mixing the two drugs will dilute the concentrations of each, they are diluted evenly and still maintain an equipotent ratio. We did not want to increase the concentrations of the mixture due to concerns that the higher concentration might result in a denser motor blockade. Prior to the start of the study, 75 cards were created—25 cards per group— and placed sealed envelopes to assure proper blinding. Each envelope was assigned a number of 1–75 by computer-based randomization.
Epidural placement occurred at the time of maternal request. Since all study participants were admitted for induction of labor, every patient received an oxytocin infusion starting at 4 mU/min for her induction. Because maternal request was the only requirement for epidural placement, the progress of labor (i.e. cervical dilation, effacement, etc.) was not recorded for this study. Each patient received standard monitoring and an approximate 500 mL bolus of crystalloid at the time of epidural placement. All epidurals were performed in the sitting position by a qualified provider at our institution (either an anesthesiology resident or faculty). The epidural catheter was threaded and secured 5 cm past loss of resistance. A test dose of 3 mL of 1.5% lidocaine with 1:200,000 epinephrine was administered to rule out intravascular or intrathecal placement. Once a negative test dose was confirmed, the patient was placed in the supine position with a wedge placed under her right hip to allow for left uterine displacement. At that point, the anesthesia provider who placed the epidural unsealed the envelope and administered the assigned medication in 3 mL increments over 3 min via the epidural catheter. Once the bolus medication was completed, the study investigator was allowed into the room to begin the data collection. Both the study investigator and the patient were blinded to the study medication.
Data were collected on an IRB-approved data collection sheet, and then transferred to our secure, password-protected departmental research drive. Data collected included: basic patient demographics, sensory level to pinprick, degree of motor blockade as determined by a modified Bromage scale,5 maternal heart rate, maternal blood pressure, need for vasopressor (phenylephrine or ephedrine) therapy, and fetal heart rate. We used the modified Bromage scale where: 0 = the ability to maintain a leg lift for prolonged periods; 1 = the ability to lift legs briefly; 2 = the ability to bend knees; 3 = the ability to wiggle toes; and 4 = no movement in the lower extremities.5
Time 0 was determined to be at the completion of the epidural bolus of the study drug. For 20 min, in 5 min intervals, the patient was assessed by the investigator and data were recorded. Vasopressor agents were administered if the blood pressure decreased below 20% of baseline or if the patient experienced signs or symptoms of hypotension. Monitoring for the study concluded after 20 min. The patient’s epidural catheter was then connected to a continuous infusion of bupivacaine 1 mg/mL-fentanyl 2.5 μg/mL solution for the remainder of her labor and delivery. The primary aim of this study was to determine the time to achieve loss of sensation to pinprick at theT10 dermatome. Secondary aims included determining: (1) the peak motor blockade; (2) the largest change in mean arterial blood pressure; and (3) the need for vasopressor therapy to treat hypotension.
Statistical Methods: Sample Size Estimate
Sample size calculations were based on our primary outcome of time to achieve loss of sensation to pinprick at theT10 dermatome. To calculate our sample size, we used previously published data by Magee et al. with respect to the time of onset of sensory blockade in patients with a lumbar epidural receiving bupivacaine and a bupivacaine plus lidocaine mixture.8 With an estimated variance of 3 min and using an α = 0.05, 80% power, and a calculated effect size of f = 0.82, we calculated a total target sample size of n = 75, or n = 25 per group, would be needed to detect differences in the primary outcome using a KruskalWallis test.9
Statistical Tests
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to conduct all statistical analyses. Data are expressed as means and standard deviations (for continuous variables) or counts and percentages (for categorical variables). Analysis of variance (ANOVA) and Fisher’s exact tests, as appropriate, were used to test for any overall group differences in outcomes. For any outcomes found to significantly differ by group, two sample t-tests were used to conduct pairwise comparisons. Normality for continuous variables was assessed using probability plots; for any variables where normality could not reasonably be assumed, the Kruskal-Wallis and Wilcoxon rank sum tests were used in place of ANOVA and two-sample t-tests, respectively. All tests of statistical significance were two-sided and used a significance level of 5%.
Results
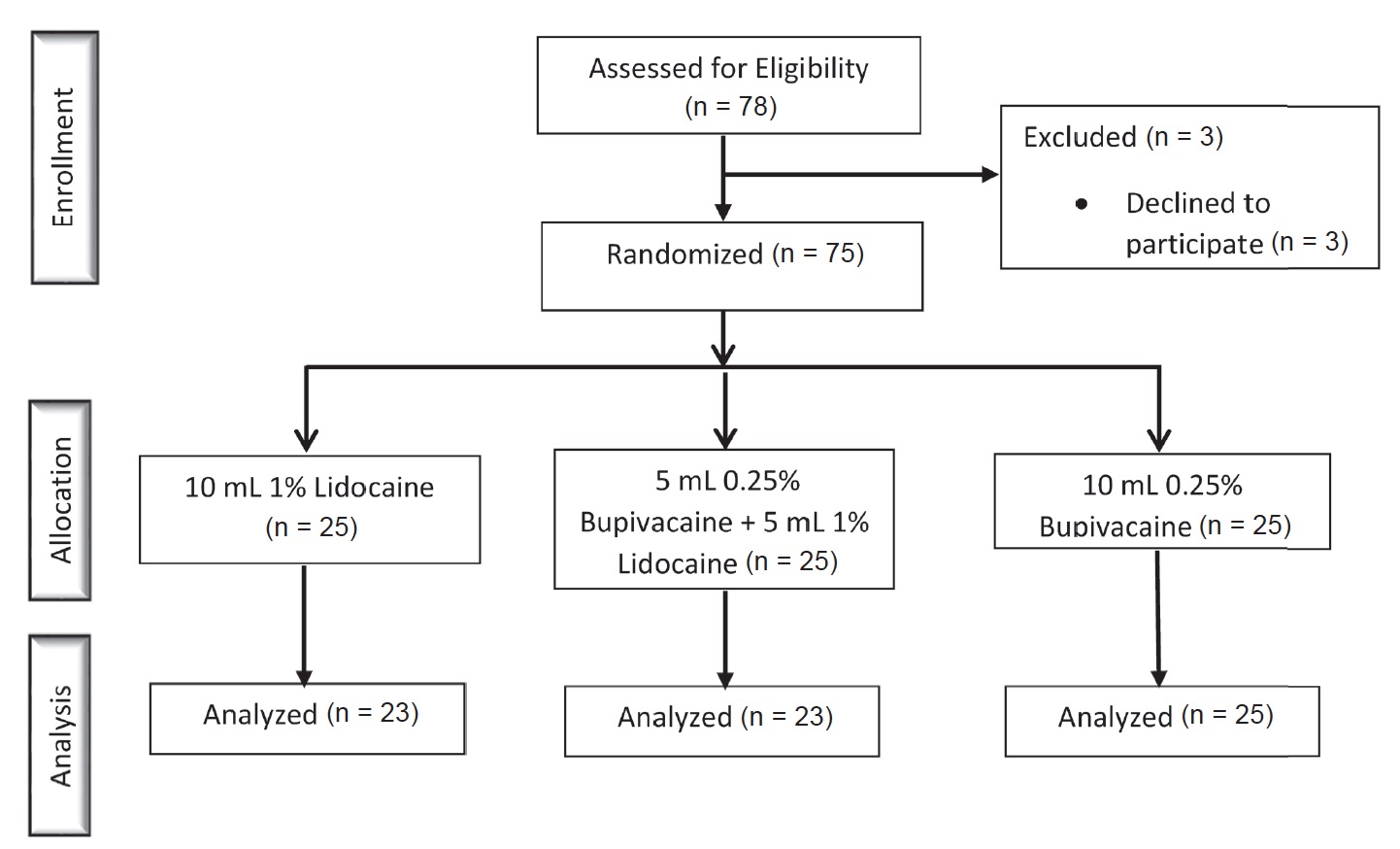
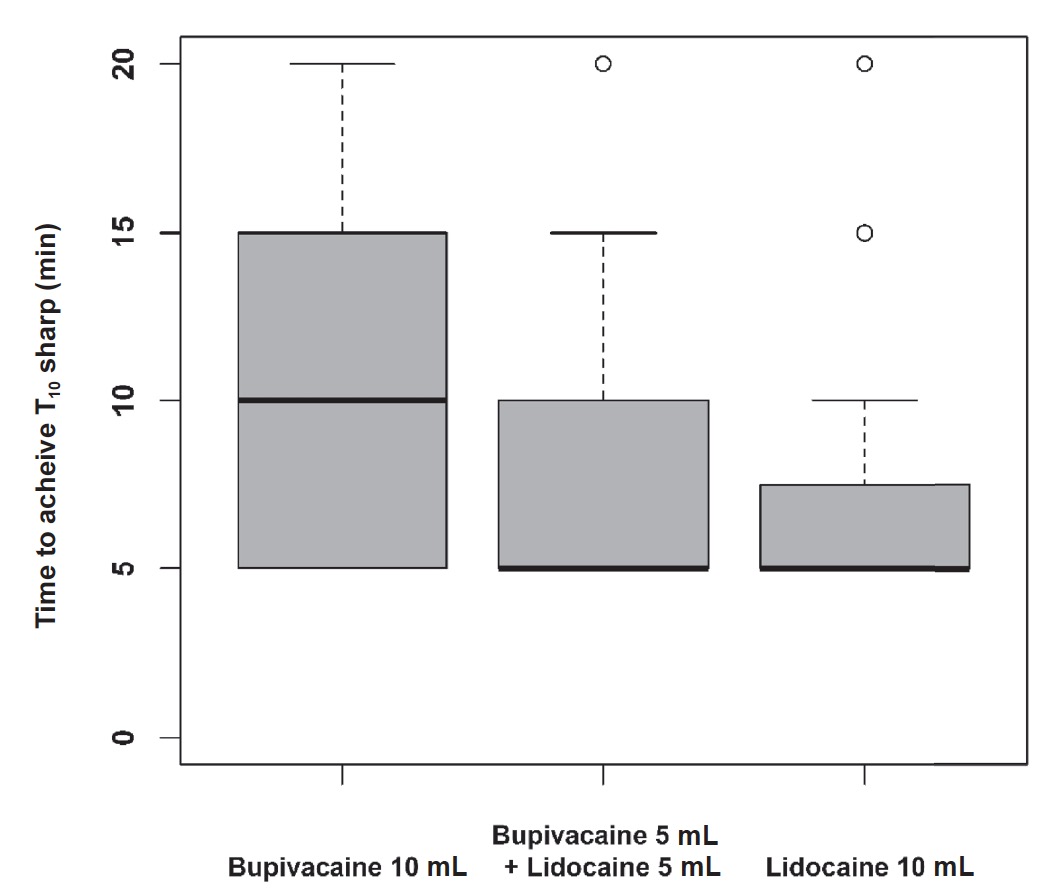
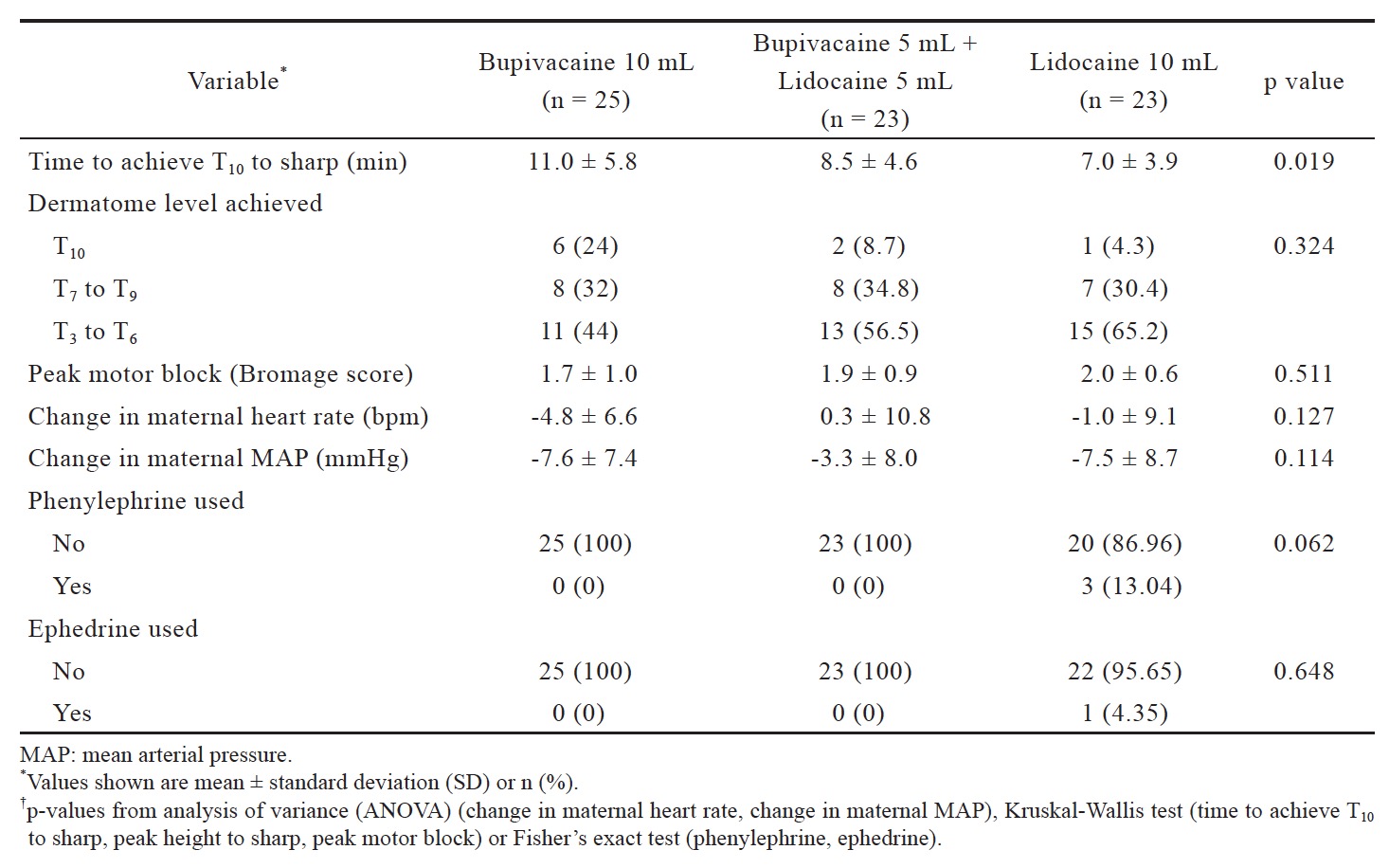
Data were analyzed on 71 of our 75 patients: B (n = 25), BL (n = 23), L (n = 23). Three patients were excluded because their epidural catheters were deemed to be non-functional after the patients did not achieve a sensory level after 20 min. One patient was excluded because she developed supraventricular tachycardia during placement of the epidural catheter (Fig. 1). Patient demographics are listed in Table 1. Time to achieve loss of sensation to pinprick at the T10 dermatome significantly differed by group (p = 0.019) (Fig. 2 and Table 2). Pairwise comparisons using the Wilcoxon rank sum test revealed that time to loss of sensation to pinprick at the T10 dermatome in the B group was significantly longer than the L group (p = 0.006), but the time to loss of sensation to pinprick at the T10 dermatome did not significantly differ in the BL group when compared to both the B (p = 0.114) as well as the L (p = 0.203) groups. Phenylephrine was used in 3 (13.0%) patients in the L group but not for any patients in the L or BL groups. However, this difference in rates of phenylephrine usage did not meet statistical significance (p = 0.062). One of these three patients also received an ephedrine bolus. There were no significant differences observed for any other secondary outcomes (Table 2).
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Discussion
Epidural analgesia is an effective method for controlling pain associated with labor and delivery.1,10 Providing rapid pain relief with minimal side effects in laboring patients via epidural analgesia is very important both for maternal satisfaction as well as preparing the mother for a safe delivery. Currently, no local anesthetic can effectively accomplish both of these goals. We designed this study to assess two commonly used LA—bupivacaine and lidocaine—both as a single drug as well in combination to determine effective onset of action for labor analgesia as determined by achieving loss of sensation to pinprick at the T10 dermatome. The concentrations and amounts we used of each local anesthetic were derived from common dosages administered for labor epidural activation.11 We chose not to add routinely-used adjuncts that either hasten onset (sodium bicarbonate) or increase the quality of the block (fentanyl) because we wanted to study the effects of the LA only.
We found a significant difference in time between the L and B groups, in that the onset of action was much faster in the L group (7.0 min) versus the B group (11.0 min). This fi nding is well-supported in the literature.2 When we compared the L and B groups to the combined (BL) group, we found that the onset of the BL group (8.5 min) was between that of the individual LA. Although mixing of the two LA did alter the time of onset—BL was 1.5 min slower than L and 2.5 min faster than B—the times did not reach statistical signifi cance. This result is consistent with fi ndings from other studies measuring similar, but slightly different, outcomes.6,7
An important secondary outcome of this study was the incidence of hypotension as measured by the need to administer bolus vasopressor medications (phenylephrine or ephedrine) during the first 20 min of labor epidural activation. All patients with hypotension requiring bolus vasopressor medications were in the L group. No patient in either the B or BL groups required treatment during the 20 min study period. Although this value did not reach statistical signifi cance, we still believe that our finding of only patients in the L group requiring vasopressor medications is important and is comparable to other studies with similar outcomes. Curatolo et al. found the probability of developing hypotension is higher in patients administered epidural lidocaine versus epidural bupivacaine.3 Ala-Kokko et al. demonstrated a higher incidence of maternal hypotension in patients receiving lidocaine (70%) when compared to bupivacaine (10%).4 A possible explanation for the increased hypotension is the faster onset of lidocaine leads to a more rapid sympathectomy without an appropriate time for physiologic compensation. As hypotension requiring vasopressor therapy was a secondary outcome in our study, we were likely underpowered to demonstrate a difference; however, a trend does exist that may show increased hypotension in patients receiving epidural lidocaine when compared to epidural bupivacaine.
There were two other important secondary outcomes of this study. The first was that there was not a clinically significant difference in motor blockade between any groups. Mean modified Bromage scores were approximately 2 in each group. A modified Bromage score of 2 correlates to patients being able to bend their knees.5 Preserved motor function is important during stage 2 of labor. Likely, if we had monitored motor blockade throughout labor and delivery, we would have seen a regression in motor blockade once this initial bolus was metabolized and the anesthetic level was maintained through a low-concentration local anesthetic infusion. Another important secondary outcome was the peak block height. Adequate analgesic coverage for stage 1 of labor requires a loss of sensation to pinprick at approximately the T10 dermatome. All groups achieved this level with 55% of subjects achieving a T6 level or higher. This block height should appropriately anesthetize the nerve fibers associated with stage 1 of labor.
One limitation to this study is that we did not follow patients to the time of delivery. Possible data we could have obtained from this would have been regression of the initial block, adequacy of coverage throughout labor and delivery, and patient satisfaction. While this information is important, our main goals of this study were to assess time of onset and hemodynamic stability of each of our LA. We believe that monitoring for 20 min after initial bolus was adequate time to obtain data pertinent to this study.
In conclusion, we found a significantly faster onset of labor analgesia in patients receiving a lidocaine only solution when compared to a bupivacaine only solution. Combining the two LA did not significantly alter the onset of action of either medication. Also, this mixture does provide hemodynamic stability and an adequate anesthetic level for stage 1 of labor; therefore, this combination of LA can be safely used to activate a labor epidural.
References
| 1 |
Jones L, Othman M, Dowswell T, et al.
Pain managementfor women in labour: an overview of systematicreviews.
Cochrane Database Syst Rev 2012;3:CD009234.
|
| 2 |
Berde CB, Strichartz GR.
Local anesthetics.
In: Miller RD, ed. Miller’s Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders;2015:1028-1054.
|
| 3 |
Curatolo M, Scaramozzino P, Venuti FS, Orlando A,Zbinden AM.
Factors associated with hypotensionand bradycardia after epidural blockade.
Anesth Analg1996;83:1033-1040.
|
| 4 |
Ala-Kokko TI, Alahuhta S, Arvela P, Vähäkangas K.
Maternal, fetal and placental distribution of lidocaineepinephrineand bupivacaine after epidural administrationfor cesarean section.
Int J Obstet Anesth 1998;7:82-87.
|
| 5 |
Sinatra RS, Goldstein R, Sevarino FB.
The clinical effectivenessof epidural bupivacaine, bupivacaine withlidocaine, and bupivacaine with fentanyl for labor analgesia.
J Clin Anesth 1991;3:219-224.
|
| 6 |
Lucas DN, Ciccone GK, Yentis SM.
Extending low-doseepidural analgesia for emergency caesarean section. A comparison of three solutions.
Anaesthesia 1999;54:1173-1177.
|
| 7 |
Seow LT, Lips FJ, Cousins MJ, Mather LE.
Lidocaine andbupivacaine mixtures for epidural blockade.
Anesthesiology1982;56:177-183.
|
| 8 |
Magee DA, Sweet PT, Holland AJ.
Epidural anaesthesiawith mixtures of bupivacaine and lidocaine.
Can AnaesthSoc J 1983;30:174-178.
|
| 9 |
Hodges JL, Lehmann EL.
The efficiency of some nonparametriccompetitors of the t-test.
Ann Math Statist1956;27:324-335.
|
| 10 | |
| 11 |
Wong CA.
Epidural and spinal analgesia: anesthesia forlabor and vaginal delivery.
In: Chestnut DH, Wong CA,Tsen LC, Ngan Kee WD, Beilin Y, Mhyre JM, eds. Chestnut’s Obstetric Anesthesia: Principles and Practice. 5th ed. Philadelphia PA: Elsevier Saunders; 2014: 457-517.
|