Abstract
Objective
Perioperative intravascular volume can be optimised by identifying predictors of fluid responsiveness. This study compared the estimated continuous cardiac output (esCCO) system for noninvasive measurement and an arterial pressure-based cardiac output (APCO) system for detecting exact changes in cardiac output (CO) among patients undergoing laparotomy without postural change.
Methods
This study was performed at Toho University Omori Medical Centre in Japan from April 2016 to July 2016 and included 26 adult patients undergoing elective laparotomy lasting > 2 h without postural change. We evaluated both interchangeability and dynamic trend. After entering the biometric data (age, sex, height, weight, heart rate, pulse wave transit time, and blood pressure), the esCCO device was calibrated. All patients were also monitored with the APCO system. Data were analysed and compared for 12 adult patients using Bland–Altman analysis and polar plots.
Result
The CO value obtained with esCCO was 0.75 ± 0.86 L/min (percentage error: 41%) lower than that obtained with the APCO system. Polar plotting revealed that the mean angular bias was 3.5°, and the radial limit of agreement was 28.3°.
Conclusion
This study demonstrated that data obtained using esCCO are not interchangeable with those obtained using the APCO system. The trending ability of the esCCO device was deemed good among patients undergoing laparotomy without postural change.
Keywords
estimated continuous cardiac output, arterial pressure-based cardiac output, haemodynamic monitoring, laparotomy
Introduction
Thermodilution (TD) technology remains a widely accepted reference method; however, this method is invasive and is thus not suitable for non-cardiac surgery. In contrast, arterial pressure- based cardiac output (APCO) systems have been developed and widely used to avoid risks associated with pulmonary artery catheter use. APCO systems are more widely used than is TD technology for non-cardiac surgery. The measurement of estimated continuous cardiac output (esCCO) is a non-invasive measurement method that has recently been investigated. It is based on pulse contour analysis combined with pulse wave transit time (PWTT).1,2 This method requires only the measurement of peripheral pulse oximetry, combined with non-invasive blood pressure and electrocardiography (ECG) monitoring.3 However, the accuracy, precision, and interchangeability of esCCO in comparison with other techniques have been validated, with several reports concluding that esCCO is not interchangeable with other methods for cardiac surgery or patients requiring pulmonary artery catheters.4-6 Numerous techniques have been used to report the outcomes of cardiac output (CO) validation studies. Recent studies have focused on dynamic trend analysis because the accuracy of a dynamic trend is more important clinically than is interchangeability in determining whether a device should be used. In addition, accuracy of a dynamic trend is necessary for identifying predictors of fluid responsiveness. However, accuracy of the dynamic trend of esCCO is unacceptable for cardiac surgery.1
Selection of the most appropriate tool for use in clinical settings requires an understanding of comparative studies so that the essential measurements can be balanced with the practical implications, costs, and risks. Measurement of the esCCO is non-invasive and low in cost; therefore, we could measure esCCO in low-risk patients if esCCO has good reliability (good trending) in the dynamic trend. The performance of the APCO system (Flo Trac system; Edwards Lifesciences, Irvine, CA, USA) has improved, particularly in normo- and hypodynamic conditions. A percentage error ≤ 30% achieved with the most recent software (the third generation) allows sufficiently accurate and precise CO measurements and trending for routine clinical use in normo- and hypodynamic conditions, in the absence of large changes in vascular tone.7 The APCO system is expensive and requires invasive arterial pressure measurements, while measurement of esCCO is non-invasive and is not associated with operational costs.
Use of the APCO system provides an effective means for anaesthesiologists to evaluate the intravascular volume under normo- and hypodynamic surgical conditions without large changes in vascular tone. The limit of the esCCO under normo-/hypodynamic conditions and/or vascular tone has not yet been reported. In this study, we investigated the trending ability of the esCCO device calibrated noninvasively and compared the results with those from a corresponding APCO system among patients undergoing laparotomy without postural change to determine if the APCO system was more suitable as a reference method for use in non-cardiac surgery.
Methods
Patient Selection
This study was performed at Toho University Omori Medical Centre in Japan from April 2016 to July 2016; 26 adult patients undergoing elective laparotomy lasting > 2 h without postural change were included. Patients of status 1 or 2 according to the American Society of Anesthesiologists physical status classification were included; patients with cardiac arrhythmias and those requiring a pacemaker or intra-aortic balloon pump were excluded. The study was conducted with the approval of the institutional review board of Toho University Omori Medical Centre, and each patient provided written informed consent before surgery.
Anaesthesia Management
Epidural anaesthesia was used in conjunction with general anaesthesia for all patients undergoing laparotomy. Inhalation of desflurane and continuous intravenous administration of remifentanil were maintained throughout surgery. The dose of remifentanil was adjusted to achieve sufficient analgesia.
Monitoring
ECG, pulse oximetry, rectal temperature, end-tidal carbon dioxide concentration (ETCO2) and end-tidal desflurane concentration were used for monitoring patients. In addition, arterial line catheters inserted into the radial arteries of the patients were used for invasive arterial pressure monitoring and APCO monitoring.
Data Sampling
After a patient’s condition stabilised following the start of surgery, the esCCO device was connected to an ECG monitor, an arterial pressure monitor, and a pulse oximetry system; APCO and esCCO were then measured, and esCCO calibration was performed using patient information. ECG, pulse oximetry waves, arterial blood pressure and PWTT were obtained using an HDM-3000 bedside monitor (Nihon Kohden, Tokyo, Japan). APCO was determined using APCO system version 3.0 (Flo Trac system; Edwards Lifesciences, Irvine, CA, USA). PWTT was calculated from the dynamic average data from 64 consecutive heartbeats, and esCCO was then calculated, as previously reported.8 APCO records haemodynamic variables, which were calculated by averaging data obtained at 20-s intervals. We collected data at 20-s intervals and paired each set of data (3 points per min). After sampling, we visually examined abnormal arterial pressure waveforms; when there was an abnormal waveform, data for 5 min before and after the abnormal waveform were excluded. When postural change was required, data collection was discontinued.
Statistical Analysis
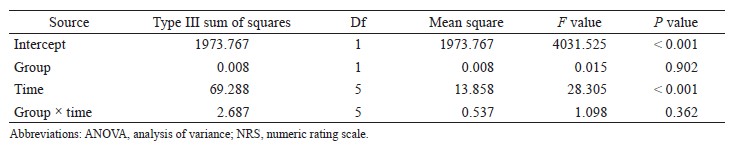
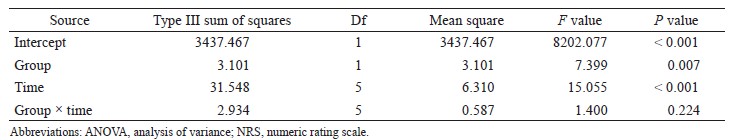
We excluded all data from patients whose systemic vascular resistance indexes (SVRIs) were not within 1,200–2,500 dyn•sec•cm-5•m2 at the time of APCO calibration. Next, we excluded all data in which SVRIs were not within 1,200–2,500 dyn• sec•cm-5•m2 from the remaining data because APCO is reliable only within the SVRI ranges (1,200–2,500 dyn•sec•cm-5•m2).9 SVRI was calculated as follows:
SVRI = (mean arterial blood pressure-central venous pressure) / cardiac index × 79.92.
Assigning CVP = 11 mmHg, we excluded data at SVRI < 1,200 dyn•sec•cm-5•m2 and assigning CVP = 4 mmHg, we excluded data at SVRI > 2,500 dyn• sec•cm-5•m2.
We performed correlation analyses of esCCO versus APCO and constructed Bland–Altman plots of esCCO versus APCO to determine bias (mean of the differences) and precision (one standard deviation of differences), thereby determining interchangeability Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA). We also performed polar plot analysis of APCO versus esCCO to determine the ability of the two continuous CO monitors to measure trends.10 The percentage error was calculated using the ratio of two standard deviations of the bias to the mean APCO and was considered clinically acceptable if that result was ≤ 30%, as proposed by Critchley and Critchley.11 Furthermore, we judged the trending ability of the esCCO system using radial limits of agreement from polar plot methodology. The radial limits of agreement were determined as follows: (1) we calculated the inclusion rates (i.e., proportion of data points that lay within the radial sector) for different radial limits (i.e., 5°, 10°, 15°,…50°); (2) we plotted the inclusion rate against the radial sector size; and (3) we determined the radial limits (°) at which the inclusion rate equalled 95% from the plot. Agreement was deemed “good trending” if the radial limits of agreement were ≤ 32°.10 Statistical significance was set at p < 0.05.
Results
Of the 26 patients initially recruited for the study, 14 were excluded (Fig. 1). Two patients were excluded because of incomplete datasets (absence of esCCO data), one patient was excluded because APCO and esCCO were not calibrated simultaneously, two patients were excluded because of inadequate datasets (absence of data for non-invasive blood pressure or PWTT at the calibration point) and 9 patients were excluded because their SVRIs were outside the reliable range (1,200–2,500 dyn•sec•cm-5•m2) at the time of APCO calibration. No patient had peripheral arterial occlusive disease.
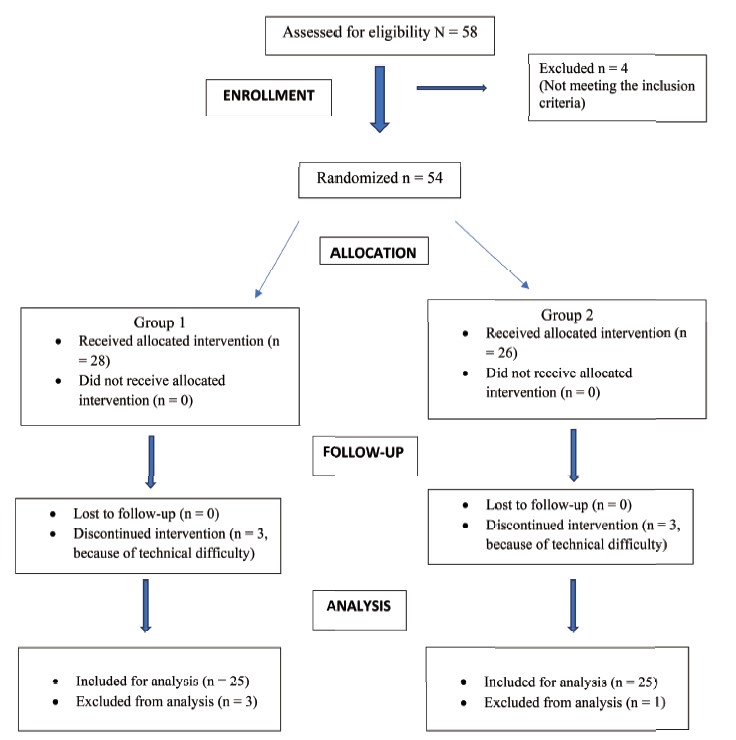
Download full-size image
APCO: arterial pressure-based cardiac output; esCCO: estimated continuous cardiac output; NIBP: non-invasive blood pressure; PWTT: pulse wave transit time; SVRI: systemic vascular resistance index.
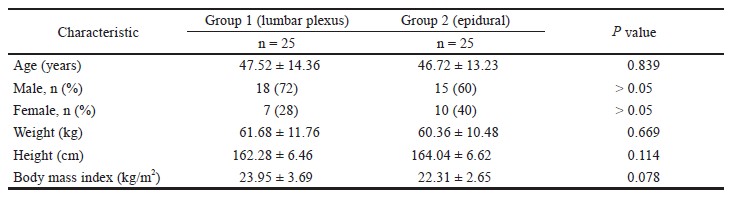
The demographic characteristics of study subjects and types of surgery are presented in Table 1. The mean age of the participants was 68.6 ± 9.6 years, and a majority of patients (8/12) were females. Four patients underwent surgery for gynaecologic malignancy and eight patients underwent gastrointestinal surgery.
Download full-size image
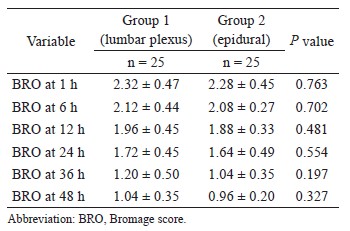
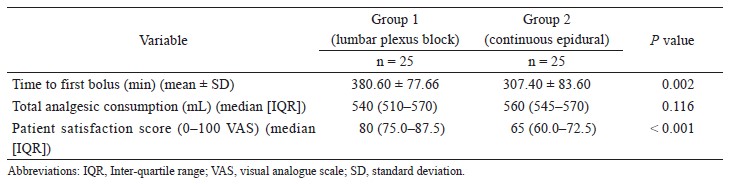
The correlation coefficient between APCO and esCCO was 0.72 (Fig. 2). Using a Bland–Altman plot to compare APCO and esCCO, we found that the bias was -0.75, the precision was 0.86 L/min and the percentage error was 41% (Fig. 3). Polar plot analysis showed that the mean angular bias was 3.5° and the radial limits of agreement were 28.3° (Fig. 4). This was in agreement with the threshold for good trending (Fig. 5).
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Discussion
In the present study, we found that the difference in CO values obtained with esCCO was 0.75 ± 0.86 L/min (percentage error: 41%) lower than those obtained with the APCO system. Polar plot analysis showed that the mean angular bias was 3.5° and that the radial limit of agreement were 28.3°. Critchley et al.10 proposed that a CO monitor is able to track changes in CO when the angular bias is < 5° and the radial limits of agreement are < 30°. This study demonstrated that the interchangeability of esCCO is not acceptable, but the trending ability of the esCCO system is good.10 Polar plots present ΔCO trend data and are a better fit with contemporary clinical use, which tend to be more concerned with dynamic patterns of CO trends following fl uid interventions rather than the absolute values. Numerous techniques have been used to report the outcomes of CO validation studies, but much like a shift from a static picture to a moving image, the preferred approach is now dynamic trend analysis.12 To identify predictors of fluid responsiveness, it is necessary to track changes in CO. The reliability of the APCO system to measure CO and track changes in CO with normal (1,200–2,500 dyn•sec•cm-5•m2) SVRI states has been verified.9 However, APCO requires invasive arterial pressure measurements and is expensive. A non-invasive, low-cost continuous CO monitoring system could be used in numerous perioperative and intensive care situations.
Joosten et al. reviewed the accuracy and precision of non-invasive CO monitoring devices in perioperative medicine.4 They reported that neither device nor technology tested was interchangeable with bolus TD.4 TD remains the most widely accepted reference method as there is no clearly established gold standard for CO measurement in human studies. 13,14 The clinical acceptability of non-invasive CO monitoring devices depends upon the context and clinical setting.12 The clinical acceptability of non-invasive CO monitoring devices during surgery is limited. Electrical bioimpedance and thoracic bioreactance methods are likely to be affected by electrical equipment and surgical procedures.15 The radial artery applanation tonometry method requires an additional sensor. The major prerequisite for arterial pressure recording is optimal positioning of the sensor over the radial artery.15 In contrast, esCCO requires only the measurement of peripheral pulse oximetry, combined with non-invasive blood pressure and ECG monitoring3 and does not require an additional sensor. Joosten et al. found that the percent error between esCCO and TD was 62% and asserted that newly developed technologies do not offer consistent reliability.4 However, several studies on esCCO have verified that the technology is interchangeable with bolus TD in cardiac surgery.16-18 To determine whether non-invasive CO monitoring devices are appropriate for use in clinical settings, we must understand their inherent measurement properties, as well as practical implications, costs, and risks. APCO, in conjunction with the most recent software, allows sufficiently accurate and precise CO measurements and trending for routine clinical use in normo- and hypodynamic conditions, in the absence of large changes in vascular tone. We were able to use APCO as a reliable CO monitoring device for patients undergoing laparotomy without severe cardiac disease because almost all surgical patients were under normo- or hypodynamic conditions. The number of patients was very low, and only 57% of the patients were included based on the characteristic of APCO. In this study, our main goal was to study the trending ability of the esCCO device calibrated noninvasively and compare the results with those from a corresponding APCO system. We can then expect the esCCO device to be used more widely than APCO. We previously reported19 that esCCO can also be used to measure CO change under hyperdynamic conditions, and under hyperdynamic conditions. In this study, the defference between the esCCO and APCO CO values increased when CO was larger than 5 L/min, this may be due to APCO accuracy under hyperdynamic conditions. Flow-related goal-directed therapy (GDT) using stroke volume (SV) change is known to improve patient outcomes20 and is particularly recommended for high-risk patients.21,22 Non-invasive flow-related GDT, based on esCCO, has potential as a valid intraoperative technique.
Although it is unknown whether SV index or cardiac index is the goal of fluid challenge using esCCO, fluid challenge is impossible unless the trending ability of the esCCO system is good. This study demonstrated that the trending ability of the esCCO device is good for patients undergoing laparotomy without postural change. In this clinical setting, non-invasive flow-related GDT, based on esCCO, has potential as a valid intraoperative technique. However, we demonstrated that, based on the trending ability of the esCCO system, further investigation is needed to determine whether the fluid responsiveness was due to SV.
Suzuki et al. showed that APCO and esCCO were significantly correlated (r = 0.62), and the bias ± precision and percentage error were 0.14 ± 1.94 (L/min) and 69%, respectively, after cardiovascular surgery. The correlation coefficient, bias ± precision and percentage error for SV variation (SVV) evaluation were 0.4, - 3.79 ± 5.08, and 99%, respectively. The time course had no effect on the biases between CO and SVV.23 Feissel et al. showed a strong correlation between esCCO and echocardiography for measuring CO and change in CO after fluid infusion in patients in intensive care units.24 We believe that clinicians may need to study other factors in addition to GDT (e.g., blood pressure and pulse pressure variation) when using esCCO to determine whether a patient is a responder or non-responder to fluid infusion; we checked the SV increase using esCCO. This study demonstrated that the trending ability of the esCCO system is good, suggesting that the esCCO system must be an effective GDT device.
The study had the following limitations:
(1) APCO is not a gold standard method of CO measurement; however, APCO is reliable within the SVRI ranges (1,200–2,500 dyn•sec•cm-5•m2).9 APCO is widely used during surgery to evaluate the intravascular volume. Slagt et al. reported that use of APCO with the most recent software allows sufficiently accurate and precise CO measurements and trending for routine clinical use in normo- and hypodynamic conditions in the absence of large changes in vascular tone, as determined by reviewing 65 manuscripts that included 2,234 patients and 44,592 observations.7 For anaesthesiologists, APCO is a gold standard method of SV. (2) We calculated as follows: SVRI = (mean arterial blood pressure-central venous pressure) / cardiac index × 79.92. (3) Assigning CVP = 11 mmHg, we excluded data at SVRI < 1,200 dyn•sec•cm-5•m2 and assigning CVP = 4 mmHg, we excluded data at SVRI > 2,500 dyn•sec•cm-5•m2. We did not use a CVP catheter because it is invasive. We hypothesised that CVP in patients during surgery is defined as 4–11 mmHg because in our previous study,19 we examined that CVP in patients during surgery was 4–11 mmHg.Conclusion
This study demonstrated that the interchangeability of esCCO is not acceptable, but the trending ability of the esCCO device is good for patients undergoing laparotomy without postural change, as determined by polar plot analysis. Further evaluation of the esCCO system is necessary, as this system involves a non-invasive technique, which may be a useful monitoring technique for patients undergoing laparotomy without postural change.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
| 1 |
Ishihara H, Sugo Y, Tsutsui M, et al.
The ability of a new continuous cardiac output monitor to measure trends in cardiac output following implementation of a patient information calibration and an automated exclusion algorithm.
J Clin Monit Comput 2012;26:465–471.
|
| 2 |
Yamada T, Tsutsui M, Sugo Y, et al.
Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output.
Anesth Analg 2012;115:82–87.
|
| 3 |
Sugo Y, Akiyama T, Takeda S, Ishihara H.
A noninvasive continuous cardiac output measurement method utilizing ECG and SpO2 pulse wave.
Jpn J Med Instrum 2005;75:63–69.
|
| 4 |
Joosten A, Desebbe O, Suehiro K, et al.
(2017) Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysis.
Br J Anaesth 2017;118:298–310.
|
| 5 |
Magliocca A, Rezoagli E, Anderson TA, Burns SM, Ichinose F, Chitilian HV.
Cardiac output measurements based on the pulse wave transit time and thoracic impedance exhibit limited agreement with thermodilution method during orthotopic liver transplantation.
Anesth Analg 2018;126:85–92.
|
| 6 |
Smetkin AA, Hussain A, Fot EV, et al.
Estimated continuous cardiac output based on pulse wave transit time in off-pump coronary artery bypass grafting: a comparison with transpulmonary thermodilution.
J Clin Monit Comput 2017;31:361–370.
|
| 7 |
Slagt C, Malagon I, Groeneveld AB.
Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation.
Br J Anaesth 2014;112:626–637.
|
| 8 |
Sugo Y, Ukawa T, Takeda S, Ishihara H, Kazama T, Takeda J.
A novel continuous cardiac output monitor based on pulse wave transit time.
Conf Proc IEEE Eng Med Biol Soc 2010;2010:2853–2856.
|
| 9 |
Suehiro K, Tanaka K, Funao T, Matsuura T, Mori T, Nishikawa K.
Systemic vascular resistance has an impact on the reliability of the Vigileo-FloTrac system in measuring cardiac output and tracking cardiac output changes.
Br J Anaesth 2013;111:170–177.
|
| 10 |
Critchley LA, Yang XX, Lee A.
Assessment of trending ability of cardiac output monitors by polar plot methodology.
J Cardiothorac Vasc Anesth 2011;25:536–546.
|
| 11 |
Critchley LA, Critchley JA.
A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques.
J Clin Monit Comput 1999;15:85–91.
|
| 12 |
Odor PM, Bampoe S, Cecconi M.
Cardiac output monitoring: validation studies—how results should be presented.
Curr Anesthesiol Rep 2017;7:410–415.
|
| 13 |
Thiele RH, Bartels K, Gan TJ.
Cardiac output monitoring: a contemporary assessment and review.
Crit Care Med 2015;43:177–185.
|
| 14 |
Squara P, Cecconi M, Rhodes A, Singer M, Chiche JD.
Tracking changes in cardiac output: methodological considerations for the validation of monitoring devices.
Intensive Care Med 2009;35:1801–1808.
|
| 15 |
Saugel B, Cecconi M, Wagner JY, Reuter DA.
Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine.
Br J Anaesth 2015;114:562–575.
|
| 16 |
Ball TR, Tricinella AP, Kimbrough BA, et al.
Accuracy of noninvasive estimated continuous cardiac output (esCCO) compared to thermodilution cardiac output: a pilot study in cardiac patients.
J Cardiothorac Vasc Anesth 2013;27:1128–1132.
|
| 17 |
Thonnerieux M, Alexander B, Binet C, Obadia JF, Bastien O, Desebbe O.
The ability of esCCOTM and ECOMTM monitors to measure trends in cardiac output during alveolar recruitment maneuver after cardiac surgery: a comparison with the pulmonary thermodilution method.
Anesth Analg 2015;121:383–391.
|
| 18 |
Wacharasint P, Kunakorn P, Pankongsap P, Preechanukul R.
Clinical validation of pulse contour and pulse wave transit time-based continuous cardiac output analyses in Thai patients undergoing cardiac surgery.
J Med Assoc Thai 2014;97(Suppl 1):S55–S60.
|
| 19 |
Terada T, Oiwa A, Maemura Y, Robert S, Kessoku S, Ochiai R.
Comparison of the ability of two continuous cardiac output monitors to measure trends in cardiac output: estimated continuous cardiac output measured by modified pulse wave transit time and an arterial pulse contour-based cardiac output device.
J Clin Monit Comput 2016;30:621–627.
|
| 20 |
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED.
Early goal-directed therapy after major surgery reduces complications and duration of hospital stay.
A randomised, controlled trial [ISRCTN38797445]. Crit Care 2005;9:R687–R693.
|
| 21 |
Bundgaard-Nielsen M, Holte K, Secher NH, Kehlet H.
Monitoring of peri-operative fluid administration by individualized goal-directed therapy.
Acta Anaesthesiol Scand 2007;51:331–340.
|
| 22 |
Donati A, Loggi S, Preiser JC, et al.
Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients.
Chest 2007;132:1817–1824.
|
| 23 |
Suzuki T, Suzuki Y, Okuda J, et al.
Cardiac output and stroke volume variation measured by the pulse wave transit time method: a comparison with an arterial pressure-based cardiac output system.
J Clin Monit Comput 2019;33:385–392.
|
| 24 |
Feissel M, Aho LS, Georgiev S, et al.
Pulse wave transit time measurements of cardiac output in septic shock patients: a comparison of the estimated continuous cardiac output system with transthoracic echocardiography.
PLoS One 2015;10:e0130489.
|