Dear Editor,
We present a case of a neonate in whom refractory hypoxia was improved by combining airway pressure release ventilation (APRV) with a cuffed tube and continuous intracuff pressure monitoring to provide steady and safe cuff sealing.
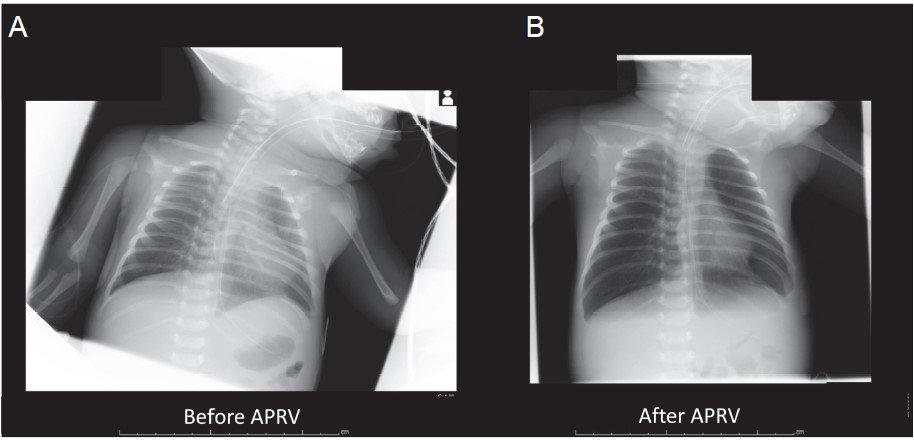
We obtained written consent from patient’s next of kin. A 21-day-old male with carbon dioxide narcosis due to congenital nasal stenosis was admitted to the intensive care unit. He measured 45 cm (height) and weighed 3.2 kg, was bone full-term by normal delivery, and was without major anomalies. Immediately after admission, his trachea was intubated with a 3.5-mm uncuffed tracheal tube (PortexTM, Smith Medical Japan Ltd., Tokyo, Japan). He was on pressure control ventilation set at a fraction of inspiratory oxygen (FiO2) of 40%, a driving pressure of 20 cm- H2O, an inspiratory interval of 0.8 s, positive end-expiratory pressure (PEEP) of 5 cmH2O, and mandatory mechanical ventilation of 25 times, which showed PaO2 = 85 mmHg and PaCO2 = 51 mmHg, probably due to widespread ground-glass opacity of lungs (Fig. 1A). Chest X-ray indicated that the tracheal tube was placed too deep, while several acoustic and fi beroptic tools showed abrogation of ventilation of one lung. Even with adjustment of the tracheal tube position, tidal volume was relatively small (< 10 mL), which required frequent recruitment maneuvers, further declining SpO2. The ventilation setting was changed to volume control ventilation; however, peak airway pressure exceeded 40 cmH2O to maintain an effective tidal volume, whose settings were compromised due to air leakage. The graphic pressure–volume curve showed that respiratory condition could be improved at a higher PEEP of 20–30 cmH2O, which was not achievable due to air leakage of the uncuffed tube at high PEEP.
Download full-size image
The patient’s uncuffed tube was replaced with a 3.0-mm cuffed tube (MICROCUFF Pediatric Endotracheal TubeTM, Halyard Health Inc., Yokohama, Japan) and APRV was applied to avoid excessively high airway pressure. We chose APRV instead of low tidal volume ventilation with high PEEP to avoid excessive peak and plateau pressure. To maintain effective cuff pressure and prevent mucosal injury, the intracuff pressure was set at 10 cmH2O and monitored by a continuous automatic cuff pressure monitor (Electronic Cuff Pressure ControllerTM, Covidien Japan, Tokyo). APRV was initiated with a high PEEP of 25 cmH2O, an inspiratory interval of 2.65 s, release of 0.35 s, and 20 cycles. His respiratory condition gradually improved (Fig. 1B) and, fi nally, his trachea was extubated on the sixth day after admission without any ventilator-associated lung injuries. After extubation, his respiratory condition was managed with only a 3.5-mm nasal airway (Koken Co., Ltd., Tokyo, Japan).
Alveolar recruitment and ventilation-perfusion matching may be optimized by APRV, which can improve oxygenation against refractory hypoxemia.1 Despite the limited experience with APRV in the pediatric population, there is increasing evidence.2 To provide adequate APRV, we must provide airway pressure with steady seal of a tracheal tube, achievable using cuffed tracheal tubes, though these are not designed for the pediatric population. A recently introduced sophisticated pediatric tracheal tube with an ultrathin cuff3 was used in the patient in this report, and it is plausible that this may have played a pivotal role in providing APRV and improving his lung condition. Additionally, cuffs used without pressure control devices can cause airway damage;4 therefore, we used a continuous automatic cuff pressure monitor to maintain a steady and safe cuff pressure. Reportedly, fluctuations in intracuff pressure may occur with lapse of time and during change in body position in a pediatric population,5 which supports the need for continuous monitoring of intracuff pressure.5 Therefore, the safe implementation of APRV with a cuffed tube in this patient may be partially attributed to the continuous automatic cuff pressure monitor.
References
| 1 |
Habashi NM.
Other approaches to open-lung ventilation: airway pressure release ventilation.
Crit Care Med 2005;33(3 Suppl):S228–S240.
|
| 2 |
Kawaguchi A, Guerra GG, Duff JP, Ueta I, Fukushima R.
Hemodynamic changes in child acute respiratory distress syndrome with airway pressure release ventilation: a case series.
Clin Respir J 2015;9:423–429.
|
| 3 |
Weiss M, Dullenkopf A, Gysin C, Dillier CM, Gerber AC.
Shortcomings of cuffed paediatric tracheal tubes.
Br J Anaesth 2004;92:78–88.
|
| 4 |
Wang H, Gao X, Liu C, et al.
Surfactant reduced the mortality of neonates with birth weight ≥1500 g and hypoxemic respiratory failure: a survey from an emerging NICU network.
J Perinatol 2017;37:645–651.
|
| 5 |
Kako H, Goykhman A, Ramesh AS, Krishna SG, Tobias JD.
Changes in intracuff pressure of a cuffed endotracheal tube during prolonged surgical procedures.
Int J Pediatr Otorhinolaryngol 2015;79:76–79.
|