Abstract
Postoperative delirium (POD) is a condition characterized by cerebral dysfunction or failure and associated with high morbidity and mortality, prolonged intensive care unit and hospital stay, increased costs and long-term disability. The risk factors can be divided into three categories: preoperative, intraoperative, and postoperative. POD is underrecognized, underdiagnosed, and undertreated condition which can lead to potentially life-threatening conditions. Prevention and treatment of POD include adequate perioperative pain control, maintenance of optimal blood pressure, water-electrolyte balance, hypoglycemia, hyperglycemia, sleep hygiene. Despite POD has been extensively studied in various types of surgery, there is not enough evidence on POD in intracranial neurosurgery. Patients undergoing open craniotomy might be at particular risk because on top of the above-mentioned factors, they also can have a direct neurosurgical brain injury. Future research on the POD in neurosurgical patients after intracranial interventions is needed. A bibliographic search was performed in the MEDLINE and PubMed virtual library. The following descriptors were used: POD, neurosurgery, anesthesia and POD, postoperative pain management and POD, water and electrolyte imbalance and POD, neurochemistry of POD. We included in this review original and review articles in the English language. Majority of non-neurosurgical patients have multiple risk factors for POD (preoperative, intraoperative, and postoperative); patients undergoing intracranial neurosurgery might have additional risks associated with neurosurgical pathology (brain tumor, cerebral hemorrhage, and severe traumatic brain injury) as well as neurosurgery-induced brain injury can also appear to be a contributing factor.
Keywords
postoperative delirium, neurosurgical procedure, risk factors, neurocritical care
Introduction
Delirium is defined as an “organ failure of the brain” similar to organ failure conditions of severe multi-organ complications.1 Postoperative delirium (POD) is one of the most frequent neuropsychiatric complications after surgery in elderly patients.2 By POD after intracranial neurosurgical procedure, we imply the transient and reversible mental dysfunction manifesting clinically with different neuropsychiatric abnormalities which can be caused by multiple factors including neurosurgery-related brain injury. By intracranial neurosurgical procedure, we imply any type of procedure associated with manipulation on the brain (open craniotomies—tumor removal; minimally invasive neurosurgical, interventional intra-cranial procedures, stereotactic surgery). POD occurs in the early postoperative period, associated with high postoperative complication rates, high morbidity and mortality rate, prolonged intensive care unit (ICU) and hospital stay, increased ICU-related costs and medication use, long-term disability, and loss of independence.3,4 Patients with POD have a greater risk of developing dementia and persistent cognitive dysfunction compared to the patients without delirium.5 The incidence of POD varies from 10% to 70% depending on the type of surgery, patient age (POD is more prevalent in the elderly), and presence of comorbidities.6 The elderly population is of particular concern, as a percentage of the aging population is increasing in most countries and the societal burden of this problem is expected to increase.7,8 The purpose of this review is to provide the perspective on POD after intracranial neurosurgery and to summarize the risk factors for POD in intracranial neurosurgery patients.
Methods
Search Strategy
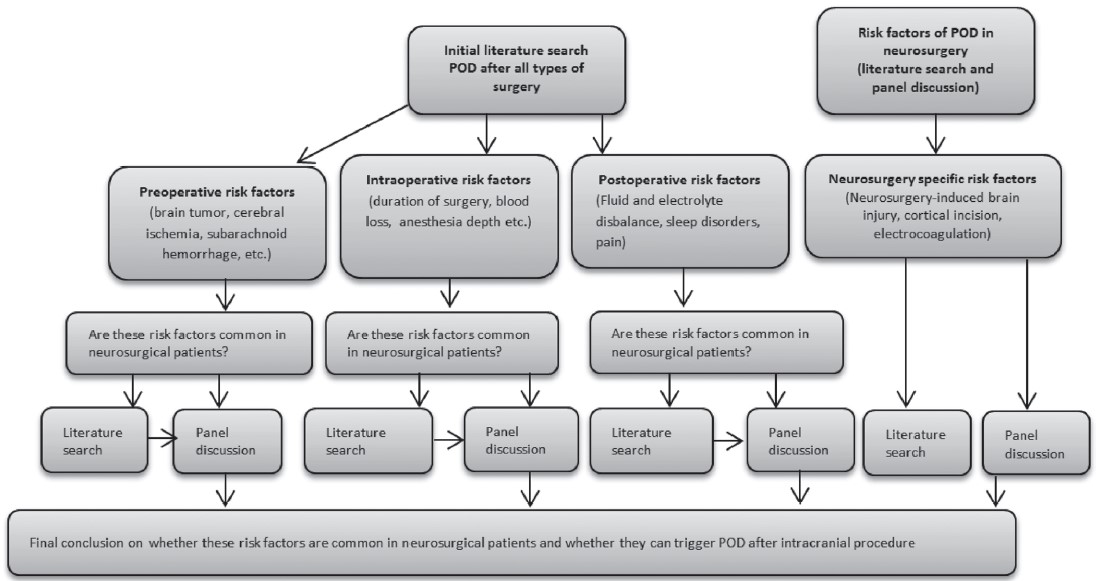
A bibliographic search was performed in the MEDLINE, PubMed, and Google Scholar (from 1976 to 2019). The following descriptors were used: (1) etiology of POD; (2) etiology of POD in neurosurgery; (3) anesthesia and POD, postoperative pain management and POD; (4) water and electrolyte imbalance and POD, water and electrolyte imbalance in neurosurgical and neurological patients; (5) electrolyte imbalance in the neurocritical care unit; (6) duration of neurosurgical vs. non-neurosurgical procedures (operations); (7) surgically-induced brain injury; (8) pathogenesis of POD. After extraction and analysis of the available risk factors for delirium, authors analyzed whether these risk factors are frequently present in neurosurgical patients (we used this method since there was no much evidence on POD in neurosurgery). Our hypothesis was that if these risk factors (cerebrovascular diseases, stroke, brain tumor, etc.) are more common among neurosurgical patients, the incidence of delirium in neurosurgical patients can be even higher (Figure 1).
Risk Factors for POD
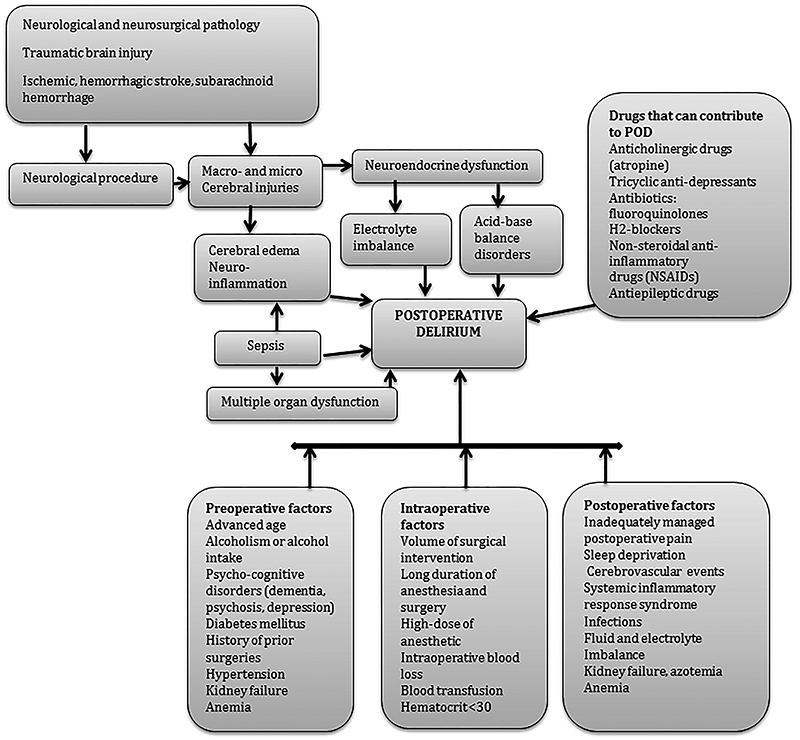
The risk factors might be divided into preoperative, intraoperative, and postoperative (Figure 2), although most patients have multiple risk factors.
Download full-size image
Preoperative Period
The most common predisposing general preoperative factors for POD are advanced age, cognitive and functional impairment, the presence of comorbidities, such as alcoholism, neurocognitive disorders (e.g., dementia, psychosis, and depression), the use of psychopharmacological agents.9,10 Other comorbid conditions that also have been associated with POD include diabetes mellitus, hypertension, fluid and electrolyte disturbances, chronic renal failure, anemia, chronic steroid treatment, and significant weight loss.11
Preoperative Risks for POD in Neurosurgical Patients
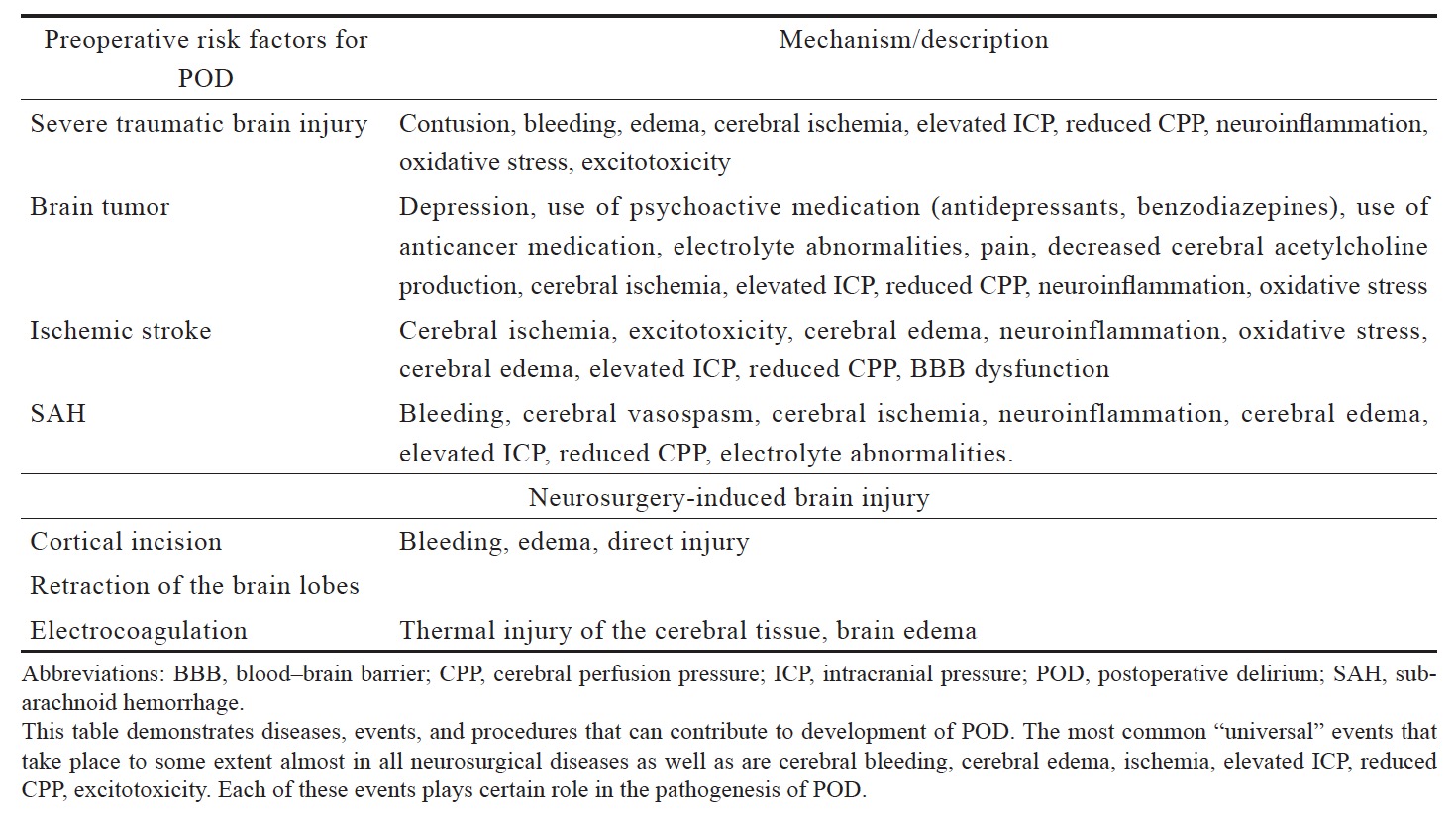
In neurosurgical patients, the spectrum of preoperative risk factors can be even greater. Thus, neurosurgical patients can have numerous neurological and neurosurgical diseases (and pathological conditions) that can contribute to the development of POD, such as acute cerebral injuries, cerebral ischemia, neoplastic brain disease, intracranial hemorrhage, subarachnoid hemorrhage (SAH), hydrocephalus, brain infection, and preexisting cognitive impairment (Table 1).12 These patients have multiple risk factors for POD even before they are admitted to the hospital and before medical and/or surgical interventions are initiated. Thus, some medical conditions such as cognitive impairment, water and electrolyte abnormalities, diseases such as brain tumors, SAH, ischemic heart disease, diabetes, hypertension, arrhythmias, and epilepsy are common in neurological and neurosurgical patients (Table 1).12,13 Many of those patients use antineoplastic drugs, antidepressants, benzodiazepines and other drugs (Table 1), which in turn can increase the risk of POD. Electrolyte disorders are also common in the preoperative period and have been shown to be the frequent systemic complications in patients with neurologic diseases.14 Among electrolyte disorders, hyponatriemia is one of the most common conditions in neurological patients15 which has been frequently reported in patients with delirium.16 Neurocognitive disorders are also commonly diagnosed in neurosurgical patients. According to Tucha et al., about 91% of patients with brain tumors demonstrated impairment in at least one area of cognition and about 71% had impairment in more than three areas of cognitive performance.17 Many patients who have indications for the intracranial procedure, also frequently have the existing disorder of cerebral physiological processes which may increase the risks of POD, such as elevated intracranial pressure, a diffuse, or regional disorder of cerebral circulation.18 It appears that the neuroanatomical location of the pathology (e.g., cortical and subcortical) also can lead to POD. It was shown that the location of intracranial hematoma within the right cerebral subcortical white matter or parahippocampus was directly associated with delirium.19
Download full-size image
Intraoperative Period
The extent of surgical intervention, duration of surgery and anesthesia, choice of anesthetic drugs, amount of intraoperative blood loss, the occurrence of hypoxemic or hypotensive events were all found to be associated with POD.20 It was found that hypotension can lead to cerebral hypoperfusion and ischemia resulting in delirium.10 Marcantonio et al. found a correlation between the degree of intraoperative blood loss, hematocrit less than 30%, and the need for blood transfusions and POD.21 They concluded that the mechanism related to a reduction of oxygen delivery to the brain during massive blood loss can induce POD.21 They also found the correlation between POD and intraoperative hypotensive as well as hypertensive events and sinus tachycardia or bradycardia.21 The perioperative blood loss and hypotension can cause inadequate brain perfusion and brain hypoxia and predispose to POD.21
Intraoperative Period in Neurosurgical Patients
Hypothetically, neurosurgical patients are at higher risk for developing cerebral ischemia or hypoperfusion than general surgical patients (taking into account that the elevated cerebral pressure and reduced cerebral perfusion are common among neurosurgical patients). Many intraoperative factors including blood loss, tissue injury, medication, and pain can activate the immune cells and trigger the production of inflammatory mediators.22 In some cases, even aseptic inflammation can be disseminated, resulting in systemic inflammation and elevated levels of cytokines in the blood which might contribute to POD.23 It has been found that the patients with delirium had higher interleukin-6 and interleukin-8 levels than those without delirium.24 Animal models have shown that peripheral injection of lipopolysaccharide activates inflammatory cascade leading to blood–brain barrier (BBB) damage, over-expression of adhesion molecules and infiltration of leukocytes into the cerebral tissue.25 It has been shown that neurosurgical patients frequently develop systemic inflammatory response syndrome.26 Thus, Boehme et al. found that as many as 20% of patients with intracerebral hemorrhage develop systemic inflammatory response syndrome.26 Apart from systemic inflammation, many other conditions such as hypoxic-reperfusion injury in stroke can lead to disruption of BBB and increase the risk of delirium.27
Surgery-Induced Brain Injury
There is still not enough evidence about POD in neurosurgical patients undergoing intracranial surgery, whether certain types of neurosurgical procedures can result in procedure-related brain injury and increase the risk of POD. Zipser et al. reported that the incidence of POD in major head and neck surgery was 32.4%.12 In fact, craniotomy is frequently associated with direct brain damage such as cortical incision, retraction of the brain lobes, electrocoagulation which can result in local bleeding and edema. However, the consequences of neurosurgery-related cerebral injury and its relations to POD have not been extensively studied yet. It appears that some neurological pathologies even without additional neurosurgery-induced brain injury can trigger POD but minimally invasive interventions such as neuroendovascular procedures lead to less prominent neurocognitive dysfunction compared to traditional neurosurgical procedures requiring craniotomy.28 Hadjivassiliou et al. in their study focusing on cognitive dysfunction after coiling or clipping of aneurismal SAH showed that both groups (with patients having their aneurisms either coiled or clipped) had impairment in all cognitive domains, compared with age matched healthy subjects; however, in the surgical group (coiling) neurocognitive outcome was poorer compared to the endovascular group (clipping), which can be explained by more severe structural damage.28
Anesthesia and POD
There are multiple anesthesia-related risk factors among neurosurgrical patients that potentially can influence POD. It was found that deep sedation during surgical procedures under regional anesthesia can increase risk of POD.21 It has also been demonstrated that light propofol-based sedation during spinal anesthesia was associated with a decrease of POD prevalence by 50% compared to deep propofol sedation.29 Moreover, prolonged administration of anesthetics and deeper (than needed) level of anesthesia can also contribute to POD.30 Furthermore, it was found that propofol-based compared to sevoflurane-based anesthesia is associated with a lower incidence of POD in elderly patients.31 Opioids have been also found to be implicated with POD. Thus, intravenous dose-dependent fentanyl administration during the neurosurgical procedure was found to have a strong correlation with development of POD in comparison with remifentanil.32 It was shown that the duration of surgery was associated with an increased risk of POD in hip fracture patients.33 Hypercapnea, anemia, and hypothermia contribute to disruption of cerebral autoregulation.34 Major surgery increases the risks of POD which can be explained by the fact that the major surgery is associated with higher total doses of anesthetics used, the degree of surgical trauma and total blood loss. Therefore, it was recommended that in order to reduce the risks of POD, major surgeries such as hip fractures should be managed by experienced surgeons and anesthesiologists who can finish surgery quick.33 It was previously believed that anesthetic dosing during surgery does not affect the long-term outcome and the effect of the drugs is eliminated after they cleared from the body. However, nowadays more and more results show that the anesthesia-related neurocognitive dysfunction can stay for a long time after anesthesia is over and the doses of anesthetics can affect it.
It appears that the optimization of depth of anesthesia and balancing of anesthetic doses which can be achieved via bispectral index (BIS) monitoring which can potentially reduce the anesthesia-related risks of POD.34 Thus, Chan et al. demonstrated that BIS monitoring-guided anesthesia in elderly surgical patients resulted in a reduction of propofol delivery by 21% and that for volatile anesthetics by 30% and reduced risk of POD and postoperative cognitive decline.34
Since neurosurgical procedures are on average longer than non-neurosurgical, the BIS monitoring might be especially beneficial for minimizing POD via optimizing intraoperative anesthetic delivery using minimally effective concentrations of volatile anesthetics for long neurosurgical procedures.
Postoperative Period
There numerous events in the postoperative period that can trigger POD have been identified. It was observed that the degree of postoperative pain levels and types of postoperative pain management have a correlation with POD.35 It was also demonstrated that severe preoperative resting pain and postoperative pain were independently associated with a high risk for POD.35,36 However, patients who used oral opioids for postoperative management were at reduced risk of POD compared to patients who used opioids via intravenous patient-controlled analgesia.35 It has been also found that type of opioids, their cumulative dose and the method of postoperative analgesia were not associated with an increased risk of POD.36
Sleep in ICU and POD
Disorder of sleep which is extremely common among critically ill patients appears to be a risk factor for POD.37 Poor quality and abnormality of sleep, its fragmentation is widely reported among critically ill patients.38 ICU environment with presence of high intensity 24-hour working personnel, constantly activated alarms, telephones, which in turn can worsen sleep in ICU settings.39 Sedative drugs are extensively used for sedation in ICU; however, they can also contribute to sleep disorders and delirium.39
The Neurochemical Mechanisms of POD
Previously published studies demonstrated that the cholinergic system plays a conceptual role in the pathogenesis of POD.40 It has been also shown that anticholinergic drug-induced toxicity might cause delirium.41 As it has been already mentioned earlier, geriatric patients are at high risk of POD. Normally, aging-associated with a reduction of acetylcholine release and activity of muscarinic receptors.40 Thus, dopamine excess and acetylcholine deficiency play a central role in delirium.42 Since many neurosurgical patients have dysfunction in cholinergic or dopaminergic system due to primary neurological or neurosurgical diseases, those patients are at high risk for POD. Similarly, any drug administered during neurosurgical procedure and postoperative neurocritical care with anticholinergic activity, such as atropine, can be a potential trigger for POD.43 Since neurosurgical patients are more likely to have seizures and other neurological disorders, they are hypothetically more likely to take benzodiazepines and gabapentine (for management of seizures or other conditions) than non-neurosurgical patients; therefore, they can be at higher risk for POD.
Postoperative Risk Factors in Neurosurgical Patients
It has been shown that severe traumatic brain injuries cause the imbalance between catabolic and anabolic hormones and neuroendocrine disorders that can result in hyperglycemia, hyper-, hyponatriemia, and increased serum cortisol.44,45 Many of neurosurgical patients are more likely (than non-surgical patients) to develop neuro-endocrine and water-electrolyte balance disorders such as syndrome of inappropriate antidiuretic hormone secretion, diabetes insipidus, and POD as a consequence.13,44
Neuroendocrine imbalances commonly result from hypothalamic-pituitary dysfunction (HPD). HPD is a known consequence of neurosurgery as well as numerous neurosurgical pathologies diseases. Hyponatriemia is a common complication in neurosurgical patients in postoperative period.33 The most common causes of hyponatremia in the neurocritical care unit include neurosurgical pathologies, such as hypophysectomy for treatment of pituitary tumors, traumatic brain injury, and SAH.25
Management of POD
Supposedly, the best way to manage POD is its prevention. In accordance with the studies that have been conducted to date, the most promising methods of preventing POD include an adequate perioperative pain management, optimal depth of anesthesia, maintenance of acid-based balance, serum glucose, electrolytes, the hemoglobin within the normal ranges, normalization of good sleep quality, early activation, and rehabilitation.34-40 Recent systematic review and meta-analysis showed that BIS-monitoring, dexmedetomidine and antipsychotics can also reduce the rates of POD.46 In neurosurgery, minimally invasive modalities (endovascular interventions) appear to be associated with less potential to cause structural cerebral injuries and subsequently cognitive decline and delirium.
Future Directions of Research
There is very weak evidence on POD in neurosurgical patients. Future research might be focused on incidence, mechanisms, risk factors, prevention, and management of POD in neurosurgical patients. At the moment, there are numerous minimally invasive neurosurgical procedures and since it has been demonstrated that endovascular minimally invasive procedures can be more beneficial in terms of POD risk reduction due to less structural injury, future research could yield more solid evidence on this matter. The comparison of open craniotomies, endovascular interventions, and stereotactic radiosurgery (gamma knife) in terms of POD will be needed. Effective perioperative pain management (including regional anesthesia in neurosurgery) seems to be an interesting and promising research area. Since cognitive dysfunction (especially in elderly population) is becoming an important healthcare issue, another potentially attractive area for research is transition of POD to long-term postoperative cognitive decline after intracranial procedures.
Conclusion
POD is a very frequent complication in postoperative period. Majority of non-neurosurgical patients have numerous (preoperative, intraoperative, and postoperative) risk factors for POD, whereas neurosurgical patients on top of that frequently have additional risks associated with neurosurgical pathology (brain tumor, cerebral hemorrhage, and severe traumatic injury) as well as neurosurgery-induced brain injury might also appear to be a contributing factor. Surprisingly, POD has not been extensively studied in neurosurgery. High-quality prospective observational studies are warranted to establish incidence, risk factors, clinical characteristics, diagnostics and management as well as long-term consequences.
Author Contributions
Dmitriy Viderman participated in the conception and design of the study, collected the literature, prepared the tables, and wrote the manuscript. Dmitriy Viderman, Evgeni Brotfain, Federico Bilotta and Agzam Zhumadilov reviewed and edited the manuscript and approved the final version.
References
| 1 |
Caraceni A, Grassi L.
Delirium: Acute Confusional States in Palliative Medicine. 2nd ed.
New York, NY: Oxford University Press 2011.
|
| 2 |
Marcantonio ER, Juarez G, Goldman L, et al.
The relationship of postoperative delirium with psychoactive medications.
JAMA. 1994;272(19):1518–1522.
|
| 3 |
Martin BJ, Buth KJ, Arora RC, Baskett RJ.
Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study.
Crit Care. 2010;14(5):R171.
|
| 4 |
Marcantonio ER, Goldman L, Mangione CM, et al.
A clinical prediction rule for delirium after elective noncardiac surgery.
JAMA. 1994;271(2):134–139.
|
| 5 |
Kat MG, Vreeswijk R, de Jonghe JF, et al.
Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years.
Dement Geriatr Cogn Disord. 2008;26(1):1–8.
|
| 6 |
Crosby G, Culley DJ, Hyman BT.
Preoperative cognitive assessment of the elderly surgical patient: a call for action.
Anesthesiology. 2011;114(6):1265–1268.
|
| 7 |
Kukreja D, Günther U, Popp J.
Delirium in the elderly: current problems with increasing geriatric age.
Indian J Med Res. 2015;142(6):655–662.
|
| 8 |
Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M.
Postoperative delirium in the elderly: risk factors and outcomes.
Ann Surg. 2009;249(1):173–178.
|
| 9 |
Yang FM, Marcantonio ER, Inouye SK, et al.
Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis.
Psychosomatics. 2009;50(3):248–254.
|
| 10 |
Echigoya Y, Kato H.
Causes of postoperative delirium after abdominal surgery in elderly patients.
Masui. 2007;56(8):932–936. [In Japanese]
|
| 11 |
Schenning KJ, Deiner SG.
Postoperative delirium: a review of risk factors and tools of prediction.
Curr Anesthesiol Rep. 2015;5(1):48–56.
|
| 12 |
Zipser CM, Deuel J, Ernst J, Schubert M, von Känel R, Böttger S.
The predisposing and precipitating risk factors for delirium in neurosurgery: a prospective cohort study of 949 patients.
Acta Neurochir (Wien). 2019;161(7):1307–1315.
|
| 13 |
Lieb K, Selim M.
Preoperative evaluation of patients with neurological disease.
Semin Neurol. 2008;28(5):603–610.
|
| 14 | |
| 15 |
Mannesse CK, Vondeling AM, van Marum RJ, van Solinge WW, Egberts TC, Jansen PA.
Prevalence of hyponatremia on geriatric wards compared to other settings over four decades: a systematic review.
Ageing Res Rev. 2013;12(1):165–173.
|
| 16 |
Coler C, Hoffman MD, Towle G, Hew-Butler T.
Hyponatremia in an 85-year-old hiker: when depletion plus dilution produces delirium.
Wilderness Environ Med. 2012;23(2):153–157.
|
| 17 |
Tucha O, Smely C, Preier M, Lange KW.
Cognitive deficits before treatment among patients with brain tumors.
Neurosurgery. 2000;47(2):324–334.
|
| 18 |
Zabolotskikh I, Trembach N.
Goal-directed cerebral hemodynamic strategy decreases the incidence of postoperative delirium in patients with intracranial hypertension in major abdominal surgery.
Crit Care. 2015;19(suppl 1):P461.
|
| 19 |
Naidech AM, Polnaszek KL, Berman MD, Voss JL.
Hematoma locations predicting delirium symptoms after intracerebral hemorrhage.
Neurocrit Care. 2016;24(3):397–403.
|
| 20 |
Patti R, Saitta M, Cusumano G, Termine G, Di Vita G.
Risk factors for postoperative delirium after colorectal surgery for carcinoma.
Eur J Oncol Nurs. 2011;15(5):519–523.
|
| 21 |
Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH.
The association of intraoperative factors with the development of postoperative delirium.
Am J Med. 1998;105(5):380–384.
|
| 22 |
Bjornsson GL, Thorsteinsson L, Gudmundsson KO, Jonsson H Jr, Gudmundsson S, Gudbjornsson B.
Inflammatory cytokines in relation to adrenal response following total hip replacement.
Scand J Immunol. 2007;65(1):99–105.
|
| 23 |
van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE.
Time‐course of cytokines during delirium in elderly patients with hip fractures.
J Am Geriatr Soc. 2008;56(9):1704–1709.
|
| 24 |
Hofer S, Bopp C, Hoerner C, et al.
Injury of the blood brain barrier and up-regulation of ICAM-1 in polymicrobial sepsis.
J Surg Res. 2008;146(2):276–281.
|
| 25 |
Sandoval KE, Witt KA.
Blood-brain barrier tight junction permeability and ischemic stroke.
Neurobiol Dis. 2008;32(2):200–219.
|
| 26 |
Boehme AK, Hays AN, Kicielinski KP, et al.
Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage.
Neurocrit Care. 2016;25(1):133–140.
|
| 27 |
Hála M.
Pathophysiology of postoperative delirium: systemic inflammation as a response to surgical trauma causes diffuse microcirculatory impairment.
Med Hypotheses. 2007;68(1):194–196.
|
| 28 |
Hadjivassiliou M, Tooth CL, Romanowski CA, et al.
Aneurysmal SAH: cognitive outcome and structural damage after clipping or coiling.
Neurology. 2001;56(12):1672–1677.
|
| 29 |
Sieber FE, Zakriya KJ, Gottschalk A, et al.
Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair.
Mayo Clin Proc. 2010;85(1):18–26.
|
| 30 |
Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD.
Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction.
Br J Anaesth. 2013;110(suppl 1):i98–i105.
|
| 31 |
Ishii K, Makita T, Yamashita H, et al.
Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients.
J Clin Anesth. 2016;33:428–431.
|
| 32 |
Radtke FM, Franck M, MacGuill M, et al.
Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium.
Eur J Anaesthesiol. 2010;27(5):411–416.
|
| 33 |
Ravi B, Pincus D, Choi S, Jenkinson R, Wasserstein DN, Redelmeier DA.
Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair.
JAMA Netw Open. 2019;2(2):e190111.
|
| 34 |
Chan MT, Cheng BC, Lee TM, Gin T; CODA Trial Group.
BIS-guided anesthesia decreases postoperative delirium and cognitive decline.
J Neurosurg Anesthesiol. 2013;25(1):33–42.
|
| 35 |
Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM.
Postoperative delirium: the importance of pain and pain management.
Anesth Analg. 2006;102(4):1267–1273.
|
| 36 |
Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER.
The impact of postoperative pain on the development of postoperative delirium.
Anesth Analg. 1998;86(4):781–785.
|
| 37 |
Thomas ML, Sing HC, Belenky G, et al.
Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity.
Thalamus Relat Syst. 2003;2(3):199–229.
|
| 38 |
Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM.
Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?
J Trauma. 2007;63(6):1210–1214.
|
| 39 |
Boyko Y, Ørding H, Jennum P.
Sleep disturbances in critically ill patients in ICU: how much do we know?
Acta Anaesthesiol Scand. 2012;56(8):950–958.
|
| 40 |
Müller WE, Stoll L, Schubert T, Gelbmann CM.
Central cholinergic functioning and aging.
Acta Psychiatr Scand. 1991;83(S366):34–39.
|
| 41 |
Trzepacz PT.
Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine.
Semin Clin Neuropsychiatry. 2000;5(2):132–148.
|
| 42 |
Itil T, Fink M.
Anticholinergic drug-induced delirium: experimental modification, quantitative EEG and behavioral correlations.
J Nerv Ment Dis. 1966;143(6):492–507.
|
| 43 |
Hammon K, DeMartino BK.
Postoperative delirium secondary to atropine premedication.
Anesth Prog. 1985;32(3):107–108.
|
| 44 |
Rafiq MF, Ahmed N, Khan AA.
Serum electrolyte derangements
in patients with traumatic brain injury
J Ayub Med Coll Abbottabad. 2013;25(1-2):162–164.
|
| 45 |
Agha A, Rogers B, Mylotte D, et al.
Neuroendocrine dysfunction
in the acute phase of traumatic brain injury.
Clin Endocrinol (Oxf). 2004;60(5):584–591.
|
| 46 |
Janssen TL, Alberts AR, Hooft L, Mattace-Raso F, Mosk CA, van der Laan L.
Prevention of postoperative delirium in elderly patients planned for elective surgery: systematic review and meta-analysis.
Clin Interv Aging. 2019;14:1095-1117.
|