Abstract
Objective: This study compared the estimated continuous cardiac output (esCCO) system and an arterial pressure-based CO (APCO) system. The goal of this study was to evaluate the dynamic trend of the esCCO calibrated with an invasive and non-invasive method.
Methods: We retrospectively identified 12 cases with complete data for the two calibration methods. Two calibration methods were analysed and compared with APCO using polar plots.
Results: Polar plotting revealed that the mean angular bias was 10.0°, and the radial limit of agreement was 37.1° when calibrated with the invasive method, while the mean angular bias was 3.5°, and the radial limit of agreement was 28.3° with the non-invasive method.
Conclusion: This study suggested that the accuracy of a dynamic trend of esCCO may not be affected by the calibration methods, and the esCCO measurement by the non-invasive calibration method may be an effective device similar to that by the invasive calibration method.
Keywords
estimated continuous cardiac output, haemodynamic monitoring, invasive calibration, non-invasive calibration
Introduction
The estimated continuous cardiac output (esCCO) is a recently investigated, non-invasive measurement method based on pulse contour analysis combined with pulse wave transit time (PWTT).1,2 This method requires the measurement of peripheral pulse oximetry, combined with blood pressure and electrocardiography (ECG) monitoring.3
The first generation of the esCCO system required an initial calibration based on externally derived reference CO and pulse pressure (invasive method). Recently, the latter technique started utilizing patient information (non-invasive method) for initial calibration. PWTT is calculated by averaging 64 consecutive heart beats, and esCCO is calculated as the following: esCCO = K × (α × PWTT + β) × HR, where α is an experimental constant, K and β are derived from PWTT, and HR is heart rate. Each parameter is calculated according to the methods described by Sugo et al.4 HR, pulse pressure, and CO are determined at the start of the measurement for each patient. The invasive calibration method uses the CO from the arterial pressure-based CO (APCO) and pulse pressure from the invasive blood pressure measurement. On the other hand, the non-invasive calibration method uses the predictive CO from biometric data (age, sex, height, weight) and pulse pressure from a non-invasive blood pressure measurement. When esCCO is calculated using the non-invasive blood pressure measurement, pulse pressure is calculated as the following: PP = (NIBP sys - NIBP dia) × (a + b × NIBP sys + c × NIBP dia),5 where a, b, and c are experimental constants, NIBP sys is the non-invasive systolic blood pressure, and NIBP dia is the non-invasive diastolic blood pressure.
The accuracy of a dynamic trend may degrade by the addition of a formula. Therefore, we hypothesized that esCCO measured with non-invasive techniques may not provide optimal accuracy of a dynamic trend compared with invasive techniques. The goal of our study was to assess the accuracy of a dynamic trend with esCCO derived from both invasive and non-invasive blood pressure measurements in comparison with APCO in patients undergoing non-cardiac surgery.
Methods
After our previous study on esCCO measurement,6 we retrospectively identified 12 cases with full data for the two calibration methods (invasive and non-invasive). Our previous study was conducted with the approval of the institutional review board of Toho University Omori Medical Centre (HDM-X-01) and JMACCT (JMA-IIA00278), and each patient provided written informed consent before surgery. The study was performed at the Toho University Omori Medical Centre in Japan from April 2016 to July 2016; 26 adult patients undergoing elective laparotomy lasting > 2 h, without postural change, were included. Patients with American Society of Anesthesiologists physical status classification 1 or 2 were included; whereas, patients with cardiac arrhythmias and those requiring a pacemaker or intra-aortic balloon pump were excluded.6 As for anaesthesia management, epidural anaesthesia was used in conjunction with general anaesthesia for all patients undergoing laparotomy. Inhalation of desflurane and continuous intravenous administration of remifentanil were maintained throughout surgery. The dose of remifentanil was adjusted to achieve sufficient analgesia. ECG, pulse oximetry waves, arterial blood pressure, and PWTT were obtained using an HDM-3000 bedside monitor (Nihon Kohden, Tokyo, Japan). APCO was determined using the APCO system Version 3 (Flo Trac system, Edwards Lifesciences, CA, USA). PWTT was calculated from the dynamic average data from 64 consecutive heartbeats, and esCCO was then calculated, as previously reported.4 APCO records haemodynamic variables, which were calculated by averaging data obtained at 20-s intervals, which was then paired. APCO records haemodynamic variables, which were calculated by averaging data obtained at 20-s intervals. We collected data at 20-s intervals and paired each set of data (3 points per min). After sampling, we visually examined abnormal arterial pressure waveforms; when there was an abnormal waveform, data for 5 min before and after the abnormal waveform were excluded. We excluded all data from patients whose systemic vascular resistance indexes (SVRIs) were not within 1200–2500 dyn•s•cm-5•m2 at the time of APCO calibration and other measurement points because APCO is reliable only within the abovementioned SVRI range.7 SVRI was calculated as follows: SVRI = (mean arterial blood pressure – central venous pressure) / cardiac index × 79.92. Assigning central venous pressure (CVP) = 11 mmHg, we excluded data at SVRI < 1200 dyn•sec•cm-5•m2 and assigning CVP = 4 mmHg, we excluded data at SVRI > 2500 dyn•sec•cm-5•m2, as in our previous study.6 The invasive and non-invasive calibration methods were analysed and compared with APCO using polar plots using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA).
Results
The mean age of the participants was 68.6 ± 9.6 years and majority of the patients (8/12) were female. Four patients underwent surgery for gynaecological malignancy (ovarian cancer 2, uterine cancer 2) and 8 patients underwent gastrointestinal surgery (duodenal cancer 2, cecal cancer 1, pancreatic cancer 3, gastric cancer 1, ileus 1).
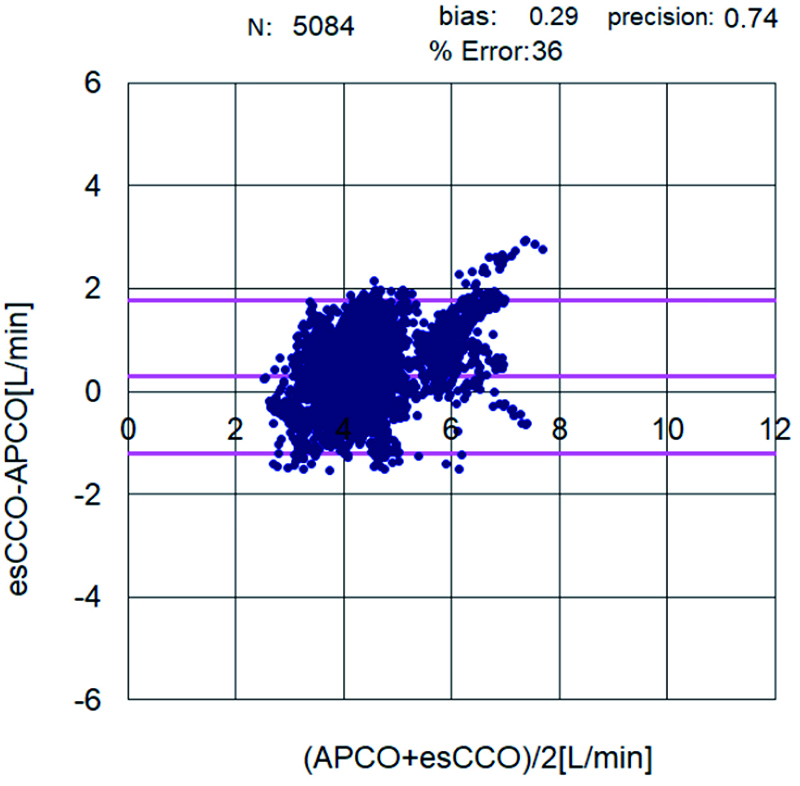
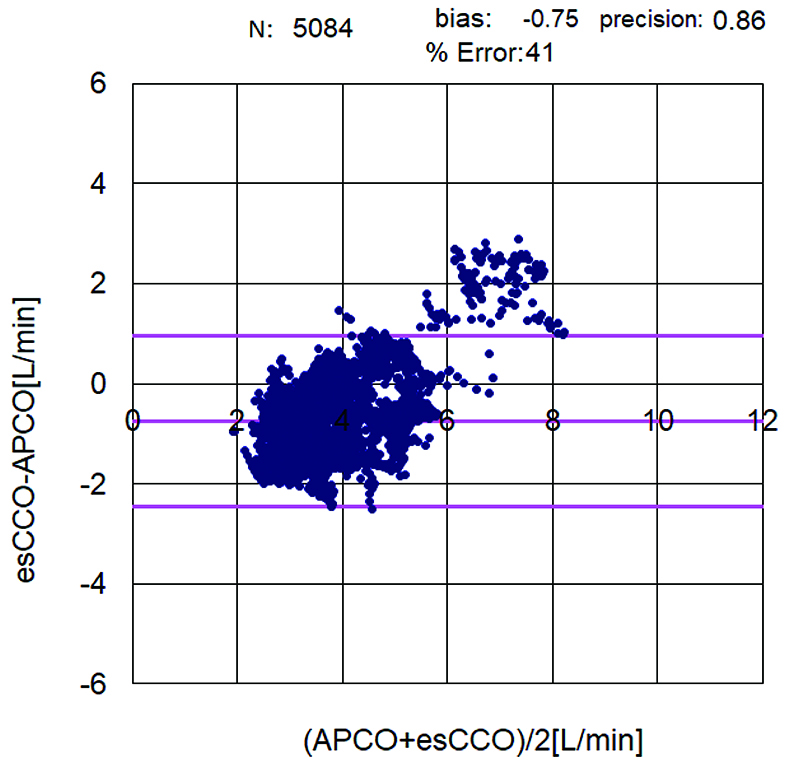
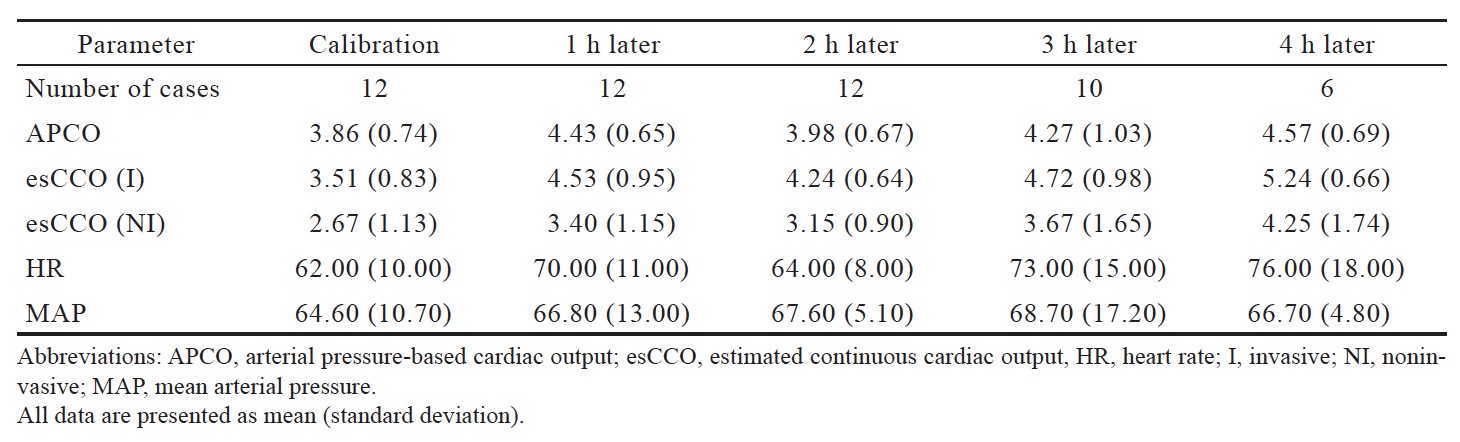
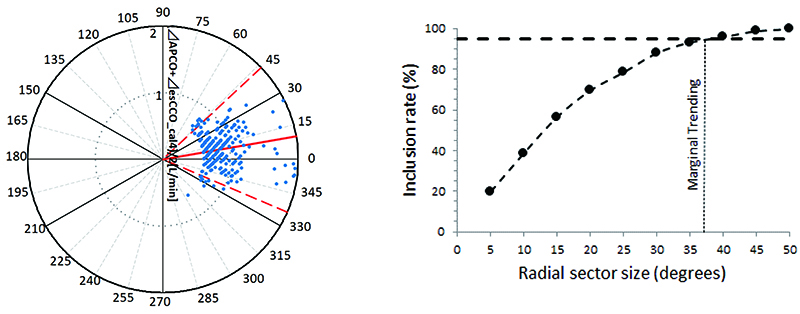
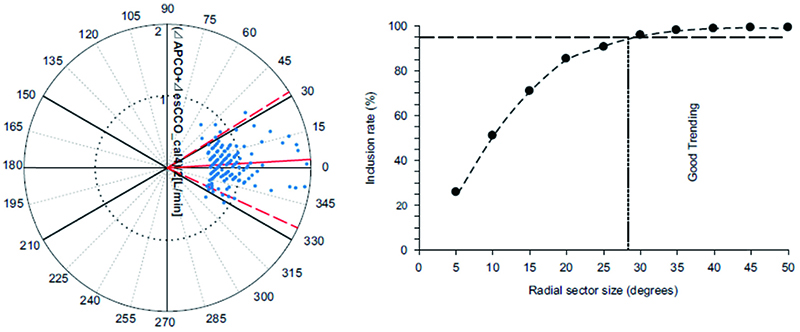
The correlation coefficient between APCO and esCCO (with invasive methods) was 0.66 and that between APCO and esCCO (with non-invasive methods) was 0.72. Using a Bland–Altman plot to compare APCO and esCCO (with invasive methods), we found that the bias was 0.29, precision was 0.74 L/min, and percentage error was 36% (Figure 1). Using a Bland–Altman plot to compare APCO and esCCO (with non-invasive methods), we found that the bias was -0.75, the precision was 0.86 L/min, and the percentage error was 41% (Figure 2). APCO and esCCO underwent similar changes during surgery (Table 1). Polar plot analysis showed that the mean angular bias of esCCO (with invasive methods) was 10.0° and the standard deviation (SD) was 16.7°, with radial limits of agreement of 37.1° (Figure 3), and the concordance rate at 30.0° was 87.9%. However, the mean angular bias of esCCO (with non-invasive methods) was 3.5° and the SD was 14.2°, with radial limits of agreement of 28.3° (Figure 4), and the concordance rate at 30.0° was 96%.
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Discussion
This study evaluated the dynamic trend of the esCCO calibrated with an invasive and non-invasive method. We hypothesized that esCCO with non-invasive techniques may not provide optimal accuracy of a dynamic trend compared with invasive techniques. However, this study revealed that the accuracy of a dynamic trend did not degrade. Bland–Altman plot showed that the invasive calibration method is more accuracy than the non-invasive calibration method. The invasive calibration method uses the CO from the APCO and pulse pressure from the invasive blood pressure measurement. On the other hand, the non-invasive calibration method uses the predictive CO from biometric data and pulse pressure from a non-invasive blood pressure measurement. In brief, the invasive calibration method used the CO from APCO such that the value of esCCO is closer to the absolute the value of APCO than the non-invasive calibration method. However, esCCO is calculated as the following: esCCO = K × (α × PWTT + β) × HR. A change in esCCO is dependent upon a change in PWTT. This is common to both methods; the accuracy of a dynamic trend of esCCO may be not affected by the calibration method used.
Enhanced recovery after surgery (ERAS) has been proven to lower both the recovery time and postoperative complication rates. Perioperative and postoperative goal-directed fluid therapy (GDFT) is included in ERAS.8 Postoperative complications such as ileus are related to tissue hypoperfusion,9 and GDFT is an effective method for determining the optimal dose of fluid therapy, inotropes, and vasopressors. ERAS is an evidence based multimodal perioperative protocol focused on stress reduction and the promotion of a return to function.10 Some patients require volume therapy where goal-directed boluses of intravenous solutions (usually a colloid) aimed at maintaining central normovolaemia by utilising changes in stroke volume.11 Arterial hypotension should be treated with vasopressors when administering intravenous fluid boluses fail to improve the stroke volume significantly (stroke volume > 10%).12,13 Inotropes should be considered in patients with reduced contractility (cardiac index < 2.5 L/min) to achieve adequate oxygen delivery.13 Most devices using GDFT require the use of minimally invasive equipment such as APCO. If we obtain a non-invasive continuous stroke volume measurement, it will be possible to reduce the incidence of perioperative complications without complications associated with radial artery catheterization. Regarding GDFT using APCO, changes in stroke volume need to be monitored. A limitation to this study is the small number of cases; therefore, further studies are required to validate the findings further.
In conclusion, this study suggests that the accuracy of a dynamic trend of esCCO was not affected by the calibration method used. Therefore, esCCO may be valid for use in GDFT.
References
| 1 |
Ishihara H, Sugo Y, Tsutsui M, et al.
The ability of a new continuous cardiac output monitor to measure trends in cardiac output following implementation of a patient information calibration and an automated exclusion algorithm.
J Clin Monit Comput. 2012;26(6):465–471.
|
| 2 |
Yamada T, Tsutsui M, Sugo Y, et al.
Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output.
Anesth Analg. 2012;115(1):82–87.
|
| 3 |
Sugo Y, Akiyama T, Takeda S, Ishihara H.
A non-invasive continuous cardiac output measurement method utilizing ECG and SpO2.
ulse wave. Jpn J Med Instr.
|
| 4 |
Sugo Y, Ukawa T, Takeda S, Ishihara H, Kazama T, Takeda J.
A novel continuous cardiac output monitor based on pulse wave transit time.
Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2853–2856.
|
| 5 |
Sugo Y, Sakai T, Terao M, Ukawa T, Ochiai R.
The agreement and trend ability of a non-invasive continuous cardiac output measurement method utilizing ECG and SpO2 ulse wave against echo Doppler during exercise.
Jpn J Med Instr. 2013;83(3):273–282. [In Japanese]
|
| 6 |
Terada T, Kessoku S, Suzuki A, et al.
Comparison of the pulse wave transit time method and an arterial pressure-based cardiac output system for measuring cardiac output trends during laparotomy without postural change.
Asian J Anesthesiol. 2019;57(3):85–92.
|
| 7 |
Suehiro K, Tanaka K, Funao T, Matsuura T, Mori T, Nishikawa K.
Systemic vascular resistance has an impact on the reliability of the Vigileo–FloTrac system in measuring cardiac output and tracking cardiac output changes.
Br J Anaesth. 2013;111(2):170–177.
|
| 8 |
Melnyk M, Casey RG, Black P, Koupparis AJ.
Enhanced recovery after surgery (ERAS) protocols: time to change practice?
Can Urol Assoc J. 2011;5(5):342–348.
|
| 9 |
Bennett-Guerrero E, Welsby I, Dunn TJ, et al.
The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery.
Anesth Analg. 1999;89(2):514–519.
|
| 10 |
Patel HRH, Cerantola Y, Valerio M, et al.
Enhanced recovery after surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy?
Eur Urol. 2014;65(2):263–266.
|
| 11 |
Gan TJ, Soppitt A, Maroof M, et al.
Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery.
Anesthesiology. 2002;97(4):820–826.
|
| 12 |
Bijker JB, van Klei WA, Vergouwe Y, et al.
Intraoperative hypotension and 1-year mortality after noncardiac surgery.
Anesthesiology. 2009;111(6):1217–1226.
|
| 13 |
Feldheiser A, Conroy P, Bonomo T, et al.
Development and feasibility study of an algorithm for intraoperative goal-directed haemodynamic management in noncardiac surgery.
J Int Med Res. 2012;40(4):1227–1241.
|