Abstract
Total intravenous anesthesia (TIVA) with propofol may improve acute postoperative pain control compared to inhalational anesthesia. The objective of this review was to comprehensively update and evaluate the existing literature on the analgesic efficacy of propofol TIVA. A systemiz literature search for randomized controlled trials in adult patients was conducted in the PubMed and Cochrane CENTRAL (EMBASE source) databases up to August 2019. Clinical trials included compared propofol TIVA against inhalational isoflurane, sevoflurane, or desflurane. Only clinical trials that studied acu postoperative pain scores or analgesic consumption as a primary outcome were included. Sixteen randomized controll trials were included. Surgical procedures evaluated included: radical gastrectomy, open vein strippin breast cancer surgery, laparoscopic cholecystectomy, inguinal herniotomy, abdominoplasty, bariatric surger lumbar spine surgery, emergency neurosurgical operations, open and laparoscopic gynecological surgeries, a dental surgery. Propofol TIVA was associated with reduced postoperative pain scores and/or decreased opioid consumption in 9 out of 16 clinical trials. There was no difference in 5 clinical trials, a propofol TIVA was associated with worse analgesic outcomes in 2 trials. Propofol TIVA may improve acu postoperative analgesia after surgery, but different factors such as surgical procedures and anesthetic/analges techniques may infl uence its effectiveness.
Keywords
acute pain, general anesthesia, propofol, postoperative pain, total intravenous anesthesia
Introduction
Acute postoperative pain remains an important unresolved clinical problem. It has been reported that 80% of patients suffer from pain after surgery, and less than half of them receive adequate pain relief.1 There appears to have been little improvement in acute postoperative pain control from patient perspective over the years.2 Undesirable outcomes including reduced patient satisfaction, delayed recovery, development of chronic post-surgical pain, and increased morbidity are associated with poor acute postoperative pain control.3-5 Therefore, techniques to improve acute postoperative pain management are needed.
Propofol is a commonly used intravenous anesthetic drug. Total intravenous anesthesia (TIVA) with propofol can be used for both induction and maintenance of general anesthesia. Propofol has been associated with pain relief both in animal studies and in human healthy volunteer studies.6-9 Therefore, the choice of general anesthetic technique may affect acute postoperative pain control. While a couple of meta-analyses comparing the analgesic efficacy of propofol TIVA versus inhalational anesthesia has been done in the past, concrete conclusions cou not be made due to discrepancy between results.10,11 Since then, numerous additional clinical trials have been published in this area. A comprehensive review of the current literature would provide clinical information that could better inform clinicians about the analgesic effect of propofol TIVA for postoperative pain. This may infl uence the choice of general anesthetic technique.
In this study, an updated scoping review of randomized controlled trials was performed to evaluate the analgesic effect of propofol TIVA for acute postoperative pain. Only clinical trials that evaluated postoperative pain scores and/or analgesic consumption as a primary outcome were included.
Methods
Search Strategy
The search methodology was conducted according to the guidelines specifi ed by the PRISMA checklist. A systematic literature search of published trials up to August 2019 was performed using the PubMed and Cochrane CENTRAL (EMBASE source) database. Only randomized controlled trials were included. The keywords used for the search were: (propofol or TIVA or “total intravenous anesthesia” or “intravenous anesthesia”), (sevofl urane or isofl urane or desfl urane or “inhalational anesthesi or “inhaled anesthetics” or “inhalational anesthetic” or “anesthetic gas”), (pain or “acute pain”), (RCT or randomized or randomised or “clinical trial” or “clinical study” or “controlled trial” or “controlled study”).
Study Selection
The total number of abstracts found in the PubMed database using the above keywords was 580 (Figure 1). Search in the Cochrane CENTRAL database yielded another 555 abstracts. After removing the abstracts that overlapped with the PubMed database, 279 abstracts from the Cochrane (EMBASE) database remained. Only trials that compared acute postoperative pain scores or analgesic consumption as the primary outcome were included. Two investigators independently reviewed the abstracts and referred to the original papers as necessary. Based on the inclusion and exclusion criteria, 110 relevant articles from PubMed, and 16 articles from Cochrane (EMBASE) were included for full-text review. Eight fulltext articles were duplicates and were excluded. After full-text review, 16 articles remained for fi nal review. Any disagreement was resolved through discussions between the two investigators.
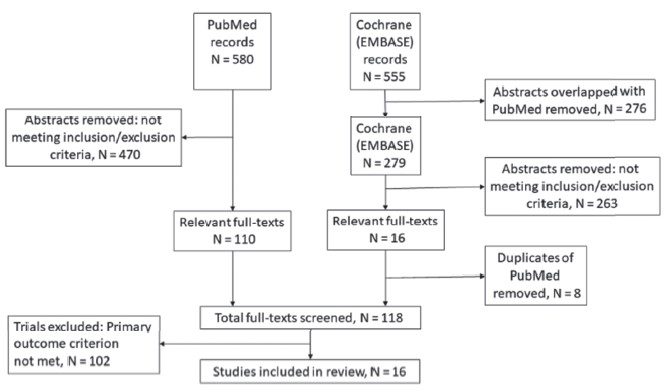
Download full-size image
The inclusion criteria were: (1) randomized controlled trial; (2) compared propofol TIVA versus inhalational anesthesia (isoflurane, sevoflurane, desflurane); (3) acute postoperative pain score or postoperative analgesic consumption was the primary outcome; (4) full articles published in English. The exclusion criteria were as follows: (1) abstract did not mention postoperative acute pain scores or analgesic consumption, (2) experimental trials on human volunteers, (3) non-randomized controlled trials, (4) studies on pediatric patients, (5) animal laboratory studies, (6) conference papers or abstracts without published articles, (7) postoperative acute pain scores and analgesic consumption were secondary outcomes, (8) the accompanying anesthetic/analgesic regime in the TIVA and inhalational anesthetic groups were different.
Clinical Trial Characteristics
The clinical trials included were randomized controlled trials that evaluated postoperative pain score or postoperative analgesic consumption as a primary outcome. General anesthetic technique used was either propofol TIVA or inhalational anesthesia with sevoflurane, desflurane, or isoflurane (GAS group). Pain scales used for measuring postoperative pain scores included the numerical rating scale, visual analog scale, and numerical analog scale. Sevoflurane was used in 11 trials; isoflurane was used in 4 trials, and desflurane was used in 4 trials. Other study variables recorded were type of surgery, perioperative analgesic consumption, and adverse events such as nausea, vomiting, and dizziness.
Results
Sixteen randomized controlled trials were included in this review. Seven clinical studies looked at general surgical procedures, 6 on gynecological procedures, 2 on orthopedic spine surgeries, and 1 on dental surgery. Seven clinical trials used intraoperative remifentanil infusion, 4 used intraoperative fentanyl (repeated bolus or infusion), 3 used nitrous oxide, and 1 used dexmedetomidine infusion. Patient controlled analgesia (PCA) opioid was used in 7 studies. Perioperative non-opioid analgesics were not used in 6 of the 11 clinical trials. There were no significant differences between TIVA and GAS groups for average age and sex distribution in the randomized controlled trials. There were also no reports of differences in American Society of Anesthesiologists (ASA) physical status and comorbidities in the clinical trials.
Nine out of the 16 clinical trials found reduced acute postoperative pain scores with propofol TIVA.12-20 Five of these clinical trials also show decreased postoperative analgesic consumption in patients given propofol TIVA (opioid in 4 studies, and diclofenac in 1 study).12-16 Five clinical studies found no difference in postoperative analgesia.21-25 In 2 clinical studies, propofol TIVA was associated with higher pain scores and higher opioid consumption.26,27
Analgesic Efficacy by Surgical Setting
General Surgery
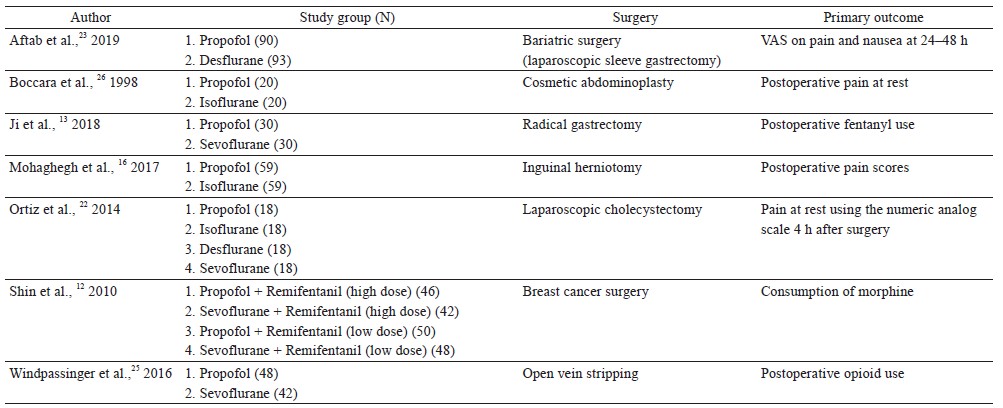
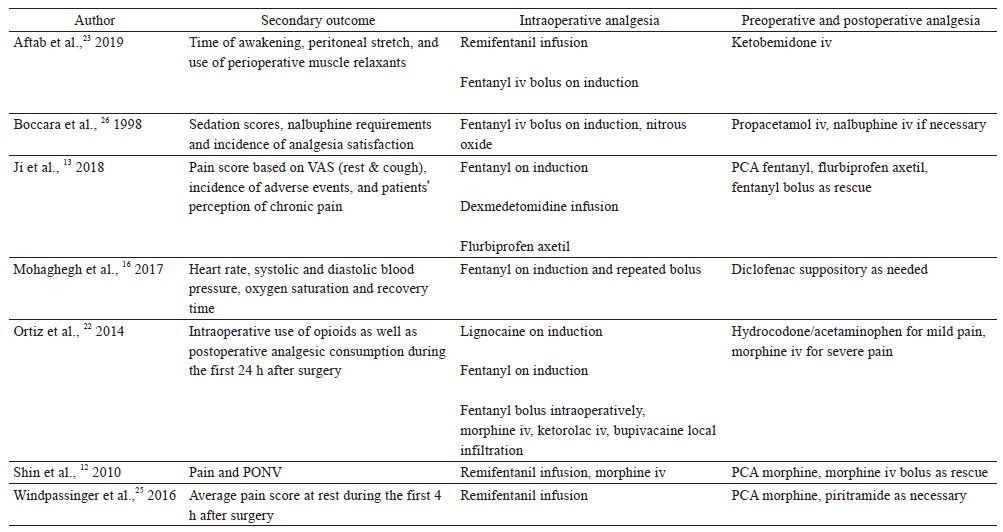
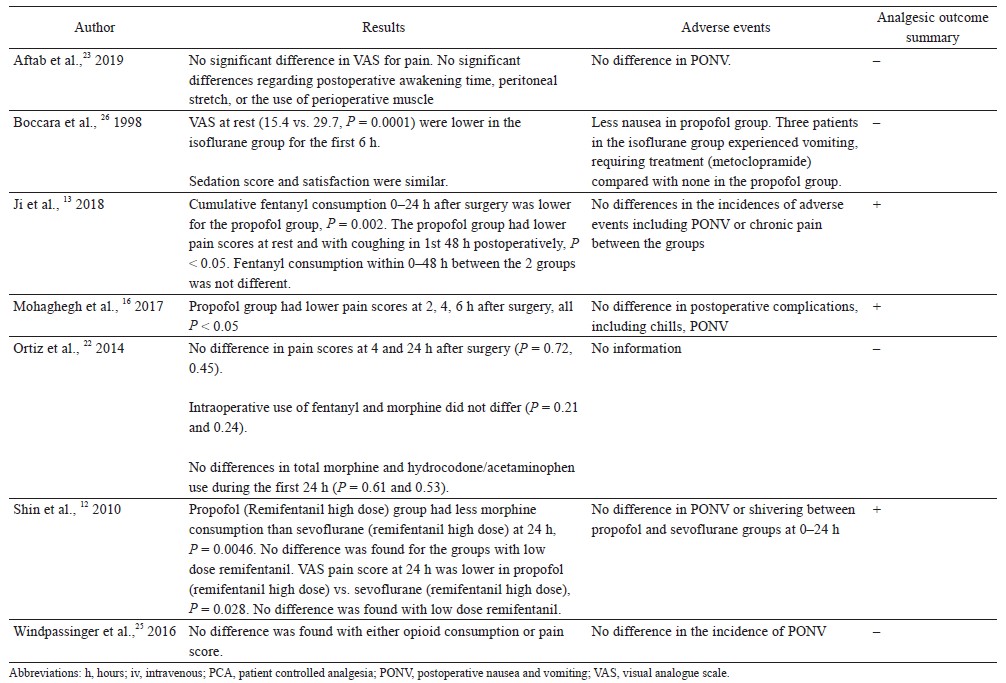
Seven randomized controlled trials compared propofol TIVA against inhalational anesthesia for acute postoperative pain control (Table 1).12,13,16,22,23,25,26 Surgical procedures studied were radical gastrectomy, laparoscopic sleeve gastrectomy, laparoscopic cholecystectomy, cosmetic abdominoplasty, breast cancer surgery, inguinal herniotomy, and open vein stripping. Three clinical trials showed better postoperative pain control with propofol TIVA, 3 studies found no difference, and 1 study showed worse analgesic outcomes.
Download full-size image
Download full-size image
Download full-size image
Ji et al. randomized 60 patients undergoing radical gastrectomy to receive propofol TIVA or sevoflurane (both groups had dexmedetomidine infusion).13 Patients in the TIVA group had lower pain scores at rest and with coughing during the first 48 hours after surgery, and they also used fewer opioids in the first 24 hours postoperatively. Mohaghegh et al. studied 102 patients undergoing inguinal herniotomy, and found reduced pain scores in the first 6 hours after surgery in patients given TIVA compared to isoflurane.16 In the clinical trial by Shin et al., 214 patients who had breast cancer surgery were randomized into 4 groups: (1) propofol TIVA with high dose intraoperative remifentanil infusion (4 ng/mL), (2) sevoflurane with high dose remifentanil infusion (4 ng/mL), (3) TIVA with low dose remifentanil infusion (1 ng/mL), and (4) sevoflurane with low dose remifentanil infusion (1 ng/mL).12 TIVA patients with high dose remifentanil infusion had significantly lower morphine consumption and pain scores in the first 24 hours after surgery compared to patients given sevoflurane with high dose remifentanil infusion. However, there were no differences in pain scores and morphine consumption between TIVA and sevoflurane patients when low dose remifentanil infusion was used.
Aftab et al. found no difference in pain scores in the first 48 hours after bariatric surgery (laparoscopic sleeve gastrectomy) in 183 obese patients who received TIVA or desflurane (both groups h remifentanil infusion).23 In a study for open vein stripping surgery, there were no differences in postoperative pain scores or opioid consumption up to postoperative day 1 between propofol TIVA and sevoflurane groups (both groups had remifentanil infusion (0.15–0.4 mcg/kg/min).25 Ortiz et al. showed no differences in postoperative analgesia between TIVA and inhalational anesthetics (isoflurane, sevoflurane, and desflurane) in 80 patients undergoing laparoscopic cholecystectomy.22 Boccara et al. compared propofol TIVA versus isoflurane (with nitrous oxide in both groups) for cosmetic abdominoplasty.26 They found that propofol TIVA was associated with worse postoperative pain scores (the first 6 hours after surgery) and higher postoperative opioid consumption.
Gynecological Surgery
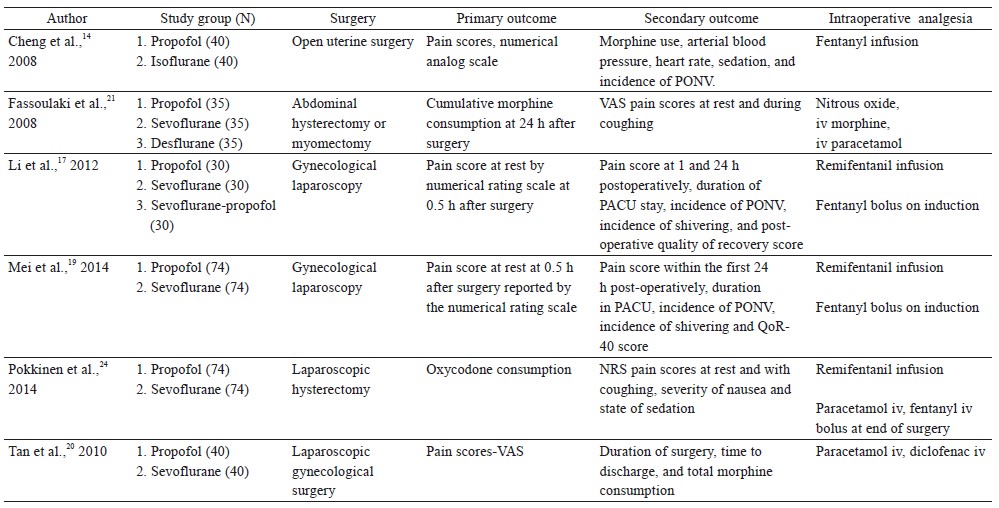
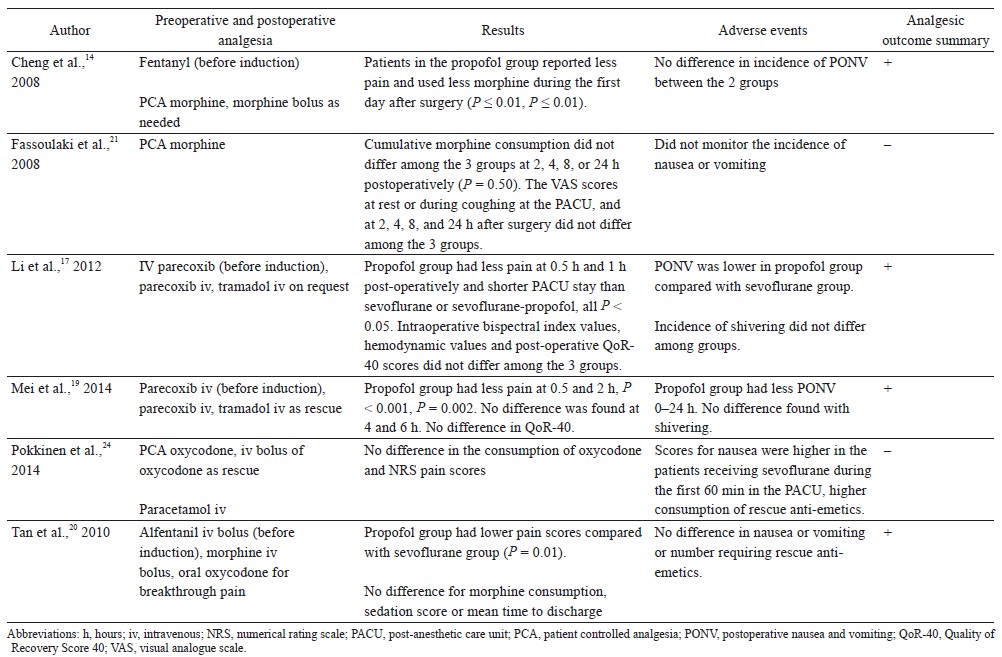
Six randomized controlled trials looked at postoperative acute pain scores or opioid consumption as the primary outcome in gynecological surgeries (Table 2).14,17,19,20,21,24 Four of the 6 randomized controlled trials found analgesic benefit with propofol TIVA, while 2 clinical trials showed no differences.
Download full-size image
Download full-size image
Li et al. randomized 90 patients undergoing laparoscopic gynecological surgery to receive propofol TIVA with remifentanil infusion (0.1 mcg/kg/min) or sevoflurane with remifentanil (0.1 mcg/kg/min).17 Propofol TIVA was associated with reduced resting pain scores at 30 minutes and 1 hour after surgery, but there were no differences at 24 hours after surgery. Pokkinen et al. compared the effect of propofol TIVA versus sevoflurane (both had remifentanil infusion 3–5 ng/mL) for laparoscopic hysterectomy in 148 patients.24 There were no differences in postoperative oxycodone consumption or pain scores. In a randomized controlled trial by Mei et al., 296 patients having gynecological laparoscopy were randomized into 4 groups using a factorial design to receive propofol TIVA or sevoflurane with or without tropisetron.19 All patients had remifentanil infusion intraoperatively (0.1 mcg/kg/min). In patients without tropisetron, propofol TIVA was associated with lower pain scores at 30 minutes and 2 hours after surgery.
In a study comparing propofol TIVA versus inhalational isoflurane (both groups had fentanyl infusion 1–2 mcg/kg/hr) for open myomectomy, patients with TIVA had lower pain scores and used less postoperative morphine in the first 24 hours after surgery.14 Tan et al. evaluated the analgesic effects of propofol TIVA versus inhalation sevoflurane in 80 patients undergoing laparoscopic gynecological day surgery.20 Propofol TIVA was associated with lower pain scores and morphine consumption after surgery. Fassoulaki et al. performed a randomized controlled trial that compared propofol TIVA, sevoflurane, and desflurane for abdominal hysterectomy or myomectomy.21 All patients were given nitrous oxide. There were no differences in postoperative pain scores and morphine consumption after surgery.
Orthopedic Surgery
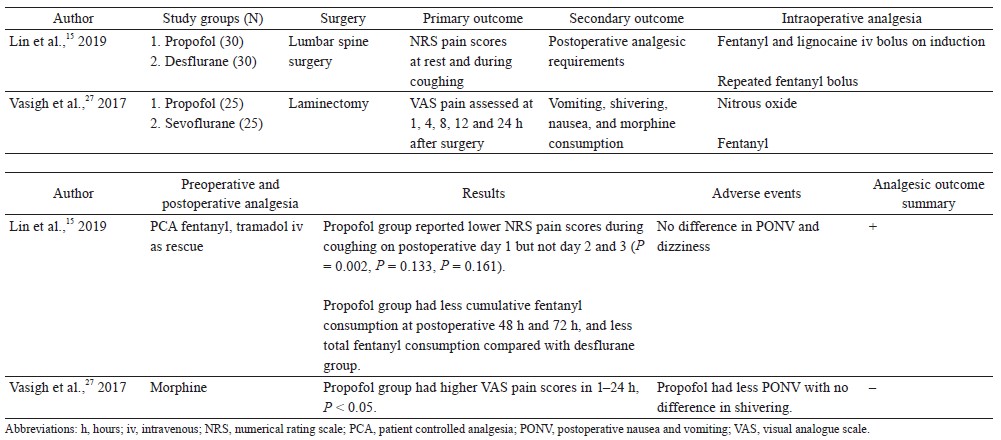
Two randomized controlled trials compared the analgesic effect of propofol TIVA versus inhalational anesthesia as a primary outcome (Table 3).15,27 One study found that propofol TIVA improved postoperative analgesia, while another study did not find any benefit.
Download full-size image
Lin et al. randomized 60 patients undergoing lumbar spine surgery to receive propofol TIVA or inhalational desflurane (both groups received intraoperative fentanyl infusion).15 Propofol TIVA was associated with lower pain scores with coughing at 24 hours after surgery and lower postoperative opioid consumption. Vasigh et al. compared 3 groups of patients undergoing lumbar disc surgery.27 Seventy-five patients were randomized to receive (1) thiopentone induction plus maintenance with sevoflurane and nitrous oxide, (2) intravenous anesthesia with propofol infusion plus nitrous oxide, (3) propofol bolus induction plus maintenance with sevoflurane and nitrous oxide. Patients induced with propofol and maintained with sevoflurane had lower pain scores and morphine consumption in the first 24 hours after surgery compared to the other 2 groups.
Others
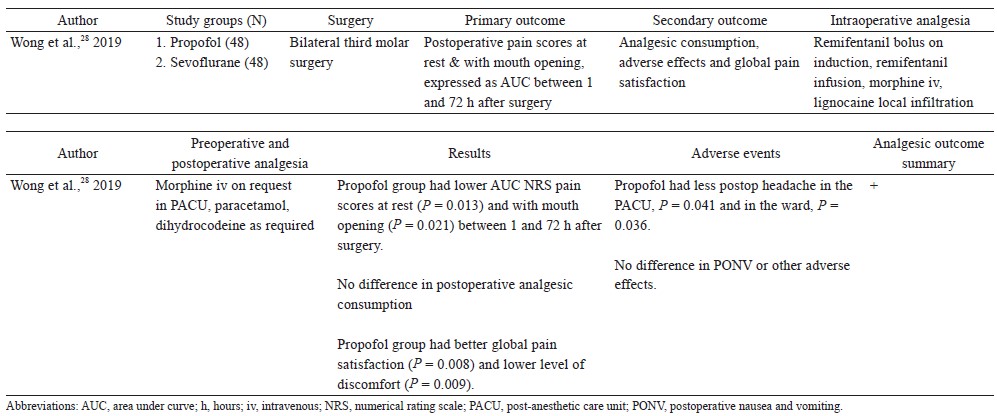
One randomized controlled trial compared the postoperative analgesic effects of propofol TIVA versus inhalational anesthetic as a primary outcome (Table 4).28 Wong et al. randomized 96 patients to receive either propofol TIVA or sevoflurane (both groups received remifentanil infusion) for bilateral third molar surgery.18 Patients anesthetized with propofol TIVA had significantly lower pain scores at rest and with mouth opening during the first 72 hours after surgery.
Download full-size image
Discussion
In this study, we reviewed and updated the current literature on the acute postoperative analgesic effects of propofol TIVA. More than half (9 out of 16) of the randomized controlled trials found that propofol TIVA reduced postoperative pain scores compared to inhalational anesthesia. Five clinical trials also showed lower postoperative analgesic consumption with propofol TIVA. Other studies found no difference, and propofol TIVA was associated with worse pain control in 2 of the clinical studies.
The results of this review suggest that propofol TIVA can improve postoperative pain control and reduce opioid consumption after surgery. However, there are also a number of negative studies. This may suggest that the analgesic effect of propofol is probably not strong. It also raises the question as to what factors influence the analgesic efficacy of propofol TIVA. These factors may include the type of surgical procedure and accompanying perioperative analgesic/ anesthetic regime. Different surgical procedures vary in postoperative pain intensity, location, and pain characteristics (nociceptive, neuropathic). Therefore, the analgesic efficacy of propofol TIVA may be different in different surgical operations, and a “one size fits all” approach for all surgical procedures may not be appropriate. Propofol has been shown to have anti- inflammatory effects, which may be one of its key analgesic mechanisms.29-31 In the dental pain model, which is a clinical model of inflammatory pain,32 propofol TIVA was associated with prolonged pain relief up to 72 hours after surgery.18 Therefore, the analgesic efficacy of propofol TIVA may be more pronounced in surgeries where postoperative inflammation is prominent.
Out of the 6 trials on gynecological surgery, all 3 studies that assessed the effect on less invasive gynecological laparoscopies found analgesic benefit with propofol TIVA.17,19,20 On the other hand, out of the 3 clinical studies that looked at relatively more invasive gynecological surgeries (laparoscopic hysterectomy, open uterine surgery, myomectomy), only 1 showed benefit with propofol TIVA.14,21,24 This may be due to differences in postoperative analgesic technique. PCA morphine was prescribed in the 3 clinical trials studying the more major gynecological surgeries, while it was not used in the trials that looked at less invasive gynecological laparoscopies. It is possible that the analgesic benefit of propofol TIVA was masked by PCA morphine. If true, this would suggest that other advanced analgesic techniques such as epidural analgesia could also attenuate and mask the analgesic benefit of propofol TIVA.
It has been suggested that the use of remifentanil may affect the efficacy of propofol TIVA. In a meta-analysis by Peng et al., the analgesic effect of propofol TIVA was greater in those with remifentanil infusion compared to those without.11 The results of the randomized controlled trial by Shin et al. for breast cancer surgery appear to support this. In the study by Shin et al, patients given propofol TIVA had lower pain scores than those given inhalational sevoflurane when a high dose of intraoperative remifentanil infusion was used.12 But there were no differences when using low dose remifentanil infusion. Remifentanil induced hyperalgesia is mediated via upregulation of N-methyl-D-aspartate (NMDA) receptors.33 Since propofol inhibits NMDA receptor activity,7,8 it has been suggested that propofol TIVA may reduce postoperative pain by reducing remifentanil-induced hyperalgesia.12 In this review, remifentanil-based anesthesia was used in 7 clinical studies. Slightly more than half of these studies (4 out of 7) showed analgesic benefit with propofol TIVA. This suggests that the use of remifentanil infusion may not be a strong factor affecting the effectiveness of propofol TIVA. This may be because a relatively high dose of remifentanil infusion for a sustained period is needed to produce clinically significant hyperalgesia,34 and such a dose is not always used in clinical practice. Another possible reason for the lack of analgesic effect in several remifentanil-based studies was the postoperative analgesic regime. Strong opioids were prescribed for postoperative pain control in the 3 studies where no difference was found (PCA morphine used in 2 studies, and intravenous ketomebedine used in one study).23-25 The analgesic efficacy of propofol TIVA may have been masked by the use of strong opioids in these 3 studies.
There were only 16 randomized controlled trials comparing propofol TIVA versus inhalational anesthesia using postoperative acute pain scores or opioid consumption as a primary outcome. Overall, there is still a relative lack of clinical evidence in this area. The heterogeneous nature of surgical procedures, anesthetic/ analgesic regime, and study designs makes it a challenge to draw concrete conclusions and perform a meta-analysis. More randomized controlled trials are needed to produce procedure-specific evidence. There is also a need to determine which analgesic/anesthetic regime works best with propofol TIVA. Opioid sparing multimodal analgesia has been advocated for optimal pain control and postoperative outcomes.1,35 However, opioid analgesics were given alone in 5 of the 16 randomized controlled trials in this review, which does not follow the current best clinical practice. Clinical trials should be performed within the context of multimodal analgesia,36 so that it would be more generalizable to real-life clinical practice.
Patient characteristics such as age and sex can affect postoperative pain control.37 There were no differences in patient characteristics including average age and sex distribution between TIVA and GAS groups in the clinical trials that were included in this review. There was also no report of differences in ASA physical status and patient comorbidities. Therefore, the patient characteristics mentioned above should not have significantly affected the results of the clinical trials.
There are some limitations to this review. Firstly, we only included randomized controlled trials where postoperative pain scores or opioid consumption was a primary outcome. More data would have been available if we also included randomized controlled trials where postoperative analgesia was a secondary outcome as well as other non-randomized controlled studies. For example, there have been a couple of retrospective case-controlled studies that showed reduced pain scores and/or opioid consumption when using propofol TIVA for liver surgery and colorectal surgery.38,39 However, these clinical studies were not included to increase the validity of our review. The second limitation was that the study sample size of the individual clinical trials was limited. None of the clinical trials had a total sample size of over 100 patients, and several trials had less than 30 patients in each study arm. Therefore, they may not be adequately powered to detect differences in pain relief or opioid consumption. A third limitation was that many studies had a short duration of observation. Out of the 16 clinical trials, only 4 trials assessed postoperative pain scores beyond 24 hours, and in 4 trials the duration of observation was less than 24 hours. Clinical trials with a longer study duration would better reflect the effect on the whole acute postoperative period. Finally, we only included randomized controlled trials that were published in English.
In conclusion, propofol TIVA can improve acute postoperative pain scores and reduce opioid consumption after surgery. Analgesic efficacy is probably small and possibly procedure-specific. However, the primary purpose of propofol TIVA is the provision of general anesthesia and acute postoperative pain can be difficult to control. Therefore, a small additional analgesic effect could still make it a useful analgesic adjunct. This should still be taken into account when choosing between general anesthetic techniques.
Author Contributions
Stanley Sau Ching Wong, Chi Wai Cheung conceptualized and designed the study. Stanley Sau Ching Wong and Wing Shing Chan acquired the data and drafted the paper. Stanley Sau Ching Wong, Wing Shing Chan, Michael G. Irwin, and Chi Wai Cheung participated in the analysis and interpretation of data and critical revision of the manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding
This study was funded by the Department of Anaesthesiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong.
References
| 1 |
Rawal N.
Current issues in postoperative pain management.
Eur J Anaesthesiol. 2016;33(3):160-171.
|
| 2 |
Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL.
Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey.
Curr Med Res Opin. 2014;30(1):149-160.
|
| 3 |
Kehlet H, Jensen TS, Woolf CJ.
Persistent postsurgical pain: risk factors and prevention.
Lancet. 2006;367(9522):1618-1625.
|
| 4 |
Rodgers A, Walker N, Schug S, et al.
Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials.
BMJ. 2000;321(7275):1493.
|
| 5 |
Beattie WS, Badner NH, Choi PT.
Meta-analysis demonstrates statistically significant reduction in postoperative myocardial infarction with the use of thoracic epidural analgesia.
Anesth Analg. 2003;97(3):919-920.
|
| 6 |
Tibbs GR, Rowley TJ, Sanford RL, et al.
HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain.
J Pharmacol Exp Ther. 2013;345(3):363-373.
|
| 7 |
Kingston S, Mao L, Yang L, Arora A, Fibuch EE, Wang JQ.
Propofol inhibits phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in neurons.
Anesthesiology. 2006;104(4):763-769.
|
| 8 |
Wong SS, Sun L, Qiu Q, et al.
Propofol attenuates postoperative hyperalgesia via regulating spinal GluN2Bp38MAPK/EPAC1 pathway in an animal model of postoperative pain.
Eur J Pain. 2018;23(4):812-822.
|
| 9 |
Bandschapp O, Filitz J, Ihmsen H, et al.
Analgesic and antihyperalgesic properties of propofol in a human pain model.
Anesthesiology. 2010;113(2):421-428.
|
| 10 |
Qiu Q, Choi SW, Wong SS, Irwin MG, Cheung CW.
Effects of intra-operative maintenance of general anaesthesia with propofol on postoperative pain outcomes—a systematic review and meta-analysis.
Anaesthesia. 2016;71(10):1222-1233.
|
| 11 |
Peng K, Liu HY, Wu SR, Liu H, Zhang ZC, Ji FH.
Does propofol anesthesia lead to less postoperative pain compared with inhalational anesthesia?: a systematic review and meta-analysis.
Anesth Analg. 2016;123(4):846-858.
|
| 12 |
Shin SW, Cho AR, Lee HJ, et al.
Maintenance anaesthetics during remifentanil-based anaesthesia migh postoperative pain control after breast cancer surgery.
Br J Anaesth. 2010;105(5):661-667.
|
| 13 |
Ji FH, Wang D, Zhang J, Liu HY, Peng K.
Effects of propofol anesthesia versus sevoflurane anesthesia on postoperative pain after radical gastrectomy: a randomized controlled trial.
J Pain Res. 2018;11:1247-1254.
|
| 14 |
Cheng SS, Yeh J, Flood P.
Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane.
Anesth Analg. 2008;106(1):264-269.
|
| 15 |
Lin WL, Lee MS, Wong CS, et al.
Effects of intraoperative propofol-based total intravenous anesthesia on postoperative pain in spine surgery: comparison with desflurane anesthesia—a randomised trial.
Medicine (Baltimore). 2019;98(13):e15074.
|
| 16 |
Mohaghegh T, Yazdi B, Norouzi A, Fateh S, Modir H, Mohammadbeigi A.
Effect of intravenous anesthesia with propofol versus isoflurane inhalation anesthesia in postoperative pain of inguinal herniotomy: a randomized clinical trial.
Med Gas Res. 2017;7(2):86-92.
|
| 17 |
Li M, Mei W, Wang P, et al.
Propofol reduces early post-operative pain after gynecological laparoscopy.
Acta Anaesthesiol Scand. 2012;56(3):368-375.
|
| 18 |
Wong SS, Leung MY, Cheung CW.
The effect of total intravenous anaesthesia with propofol on postoperative pain after third molar surgery: a double-blind randomized controlled trial.
Eur J Pain. 2019;23(5):884-893.
|
| 19 |
Mei W, Li M, Yu Y, et al.
Tropisetron alleviate early post-operative pain after gynecological laparoscopy in sevoflurane based general anaesthesia: a randomized, parallel-group, factorial study.
Eur J Pain. 2014;18(2):238-248.
|
| 20 |
Tan T, Bhinder R, Carey M, Briggs L.
Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane.
Anesth Analg. 2010;111(1):83-85.
|
| 21 |
Fassoulaki A, Melemeni A, Paraskeva A, Siafaka I, Sarantopoulos C.
Postoperative pain and analgesic requirements after anesthesia with sevoflurane, desflurane or propofol.
Anesth Analg. 2008;107(5):1715-1719.
|
| 22 |
Ortiz J, Chang LC, Tolpin DA, Minard CG, Scott BG, Rivers JM.
Randomized, controlled trial comparing the
effects of anesthesia with propofol, isoflurane, desflurane and sevoflurane on pain after laparoscopic cholecystectomy.
Braz J Anesthesiol. 2014;64(3):145-151.
|
| 23 |
Aftab H, Fagerland MW, Gondal G, Ghanima W, Olsen MK, Nordby T.
Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia: a double blind, randomized, controlled trial.
Surg Obes Relat Dis. 2019;15(9):1505-1512.
|
| 24 |
Pokkinen SM, Yli-Hankala A, Kalliomäki ML.
The effects of propofol vs. sevoflurane on post-operative pain and need of opioid.
Acta Anaesthesiol Scand. 2014;58(8):980-985.
|
| 25 |
Windpassinger M, Plattner O, Gemeiner J, et al.
Opioid use after propofol or sevoflurane anesthesia: a randomized trial.
Can J Anaesth. 2016;63(11):1258-1265.
|
| 26 |
Boccara G, Mann C, Pouzeratte Y, Bellavoir A, Rouvier A, Colson P.
Improved postoperative analgesia with isoflurane than with propofol anaesthesia.
Can J Anaesth. 1998;45(9):839-842.
|
| 27 |
Vasigh A, Najafi F, Jaafarpour M, Khajavikhan J, Khani A.
The effect of sevoflurane plus propofol on pain and complications after laminectomy: a randomized double blind clinical trial.
J Clin Diagn Res. 2017;11(4):UC05-UC08.
|
| 28 |
Wong SSC, Leung MYY, Cheung CW.
The effect of total intravenous anaesthesia with propofol on postoperative pain after third molar surgery: a double-blind randomized controlled trial.
Eur J Pain. 2019;23(5):884-893.
|
| 29 |
Ma X, Hu YW, Zhao ZL, et al.
Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1α-dependent manner.
Arch Biochem Biophys. 2013;533(1-2):1-10.
|
| 30 |
Qiu Q, Sun L, Wang XM, et al.
Propofol produces preventive analgesia via GluN2B-containing NMDA Receptor/ ERK1/2 Signaling Pathway in a rat model of inflammatory pain.
Mol Pain. 2017;13:1744806917737462.
|
| 31 |
Chen RM, Chen TG, Chen TL, et al.
Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages.
Ann N Y Acad Sci. 2005;1042:262-271.
|
| 32 |
Cheung CW, Ng KF, Choi WS, Chiu WK, Ying CL, Irwin MG.
Evaluation of the analgesic efficacy of local dexmedetomidine application.
Clin J Pain. 2011;27(5):377-382.
|
| 33 |
Zhao M, Joo DT.
Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance.
Anesthesiology. 2008;109(2):308-317.
|
| 34 |
Yu EH, Tran DH, Lam SW, Irwin MG.
Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain?
Anaesthesia. 2016;71(11):1347-1362.
|
| 35 |
Pogatzki-Zahn EM, Segelcke D, Schug SA.
Postoperative pain-from mechanisms to treatment.
Pain Rep. 2017;2(2):e588.
|
| 36 |
Joshi GP, Schug SA, Kehlet H.
Procedure-specific pain management and outcome strategies.
Best Pract Res Clin Anaesthesiol. 2014;28(2):191-201.
|
| 37 |
Yang MM, Hartley RL, Leung AA, et al.
Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis.
BMJ Open. 2019;9(4):e025091.
|
| 38 |
Wong SSC, Choi SW, Lee Y, Irwin MG, Cheung CW.
The analgesic effects of intraoperative total intravenous anesthesia (TIVA) with propofol versus sevoflurane after colorectal surgery.
Medicine (Baltimore). 2018;97(31):e11615.
|
| 39 |
Chan AC, Qiu Q, Choi SW, et al.
Effects of intra-operative total intravenous anaesthesia with propofol versus inhalational anaesthesia with sevoflurane on post-operative pain in liver surgery: a retrospective case-control study.
PloS One. 2016;11(2):e0149753.
|