Abstract
Background: General anesthesia or sedation is commonly required for pediatric patients undergoing magnetic resonance imaging (MRI) scans, and airway management dur the procedure is the highest concern for anesthesiologists owing to the limited access to patient in the MRI unit. The use of supraglottic airway devices (SADs) has recently become more popular than endotracheal tubes; however, the feasibility of using SADs for children in MRI suites reported only in a few studies that involved healthy patients.
Methods: We present a successful case series of 30 pediatric patients, and the majority are high-risk patients, including patients with aromatic L-amino acid decarboxylase (AADC) deficiency, mitochondrial disease, and tuberous sclerosis, using either i-gel or laryng masks for airway maintenance during MRI examination.
Results: A total of 38 MRI exams were conducted; the patients’ median age was 4 (range 1.6–17.0 years), and the mean examination time was 50.87 minutes. No patient experienced oxygen desaturation, and only 1 patient with AADC defi ciency had an episode of hypotension. The MRI scans were completed without interruption with an adequate image quality according to a specialized radiologist.
Conclusions: From the clinical point of view, this case series demonstrated a broader application of SADs for airway maintenance during MRI scans for pediatric patients with a high risk during anesthesia rather than only for a healthy patient population.
Keywords
airway management, aromatic L-amino acid decarboxylase deficiency, laryngeal masks, magnetic resonance imaging, pediatric
Introduction
Pediatric patients undergoing magnetic resonance imaging (MRI) scans inevitably have to receive general anesthesia or sedation because the MRI procedure is time-consuming, susceptible to motion, and depends greatly on the cooperation of the patient.1,2 General anesthesia through an endotracheal tube for airway maintenance is considered the first choice for children who are neurologically impaired, have global developmental delay, or exhibit severe disturbances of behavior to avoid hypoxemia or failed sedation during the procedure.2
Recently, the use of supraglottic airway devices (SADs) in MRI suites has become more popular because it is associated with lower airway complication rates and is less invasive.1,2 However, the feasibility and safety of using SADs during MRI scans for children who are neurologically impaired or with developmental delay have not been tested yet. In this study, we present a case series of 30 pediatric patients uncooperative to immobility because of physical reasons, such as neurological and developmental disabilities, undergoing MRI with SADs to maintain an airway.
Methods
This study was approved by the Institutional Review Board of National Taiwan University Hospital (201811074RINA), and the requirement for written informed consent was waived by the Research Ethics Committee. We retrospectively analyzed the medical records of pediatric patients with neurological impairment or developmental abnormality using SADs for airway maintenance during MRI scans at a single medical center (National Taiwan University Children Hospital, Taipei, Taiwan) between October 2016 and July 2018.
Each patient received general anesthesia in the space adjacent to the MRI room by using a combination of thiamylal (5–10 mg/kg) or propofol (2–4 mg/kg) with fentanyl (1 μg/kg). Neuromuscular blocking agent (low-dose cisatracurium [< 0.15 mg/kg]) was added based on the attending physician’s preference. A reusable Ambu laryngeal mask airway (LMA; Ambu® Aura40™, Copenhagen, Denmark) or i-gel (Intersurgical Ltd, Wokingham, Berkshire, UK) chosen at the discretion of the attending anesthesiologist was inserted on each patient after general anesthesia. Proper SADs position without leak was confirmed by the auscultation by using a stethoscope to detect possible flow leak with manual bagging pressure of 20 cmH2O. After induction, all patients, except those with aromatic L-amino acid decarboxylase (AADC) deficiency, were transferred immediately to the MRI room. Patients with AADC deficiency received a lumbar puncture in the lateral decubitus position to collect cerebrospinal fluid for genetic analysis before the MRI scan to avoid additional anesthesia for the lumbar puncture. During the MRI exam, all patients were monitored through MRI-compatible electrocardiography, pulse oximetry, noninvasive blood pressure measurements, and capnography (Invivo Precess, Orlando, FL, USA). In the MRI room, anesthesia was maintained by using sevoflurane (MAC, 0.5–1.5) delivered through an MRI-compatible anesthesia machine. A ventilator was set on volume-controlled mode with a tidal volume of 6 mL/kg ideal body weight and respiratory rate titrated to maintain end-tidal carbon dioxide at the range of 35–45 mmHg. At the end of the procedure, patients were transferred immediately to space where general anesthesia was induced outside the MRI room for the removal of SADs. In our institute, patients with AADC deficiency were routinely transferred to our intensive care unit (ICU) for overnight observation. Other patients were sent to a distant post-anesthesia care unit (PACU), which was not on the same floor as the MRI suite.
Respiratory and hemodynamic data were collected by reviewing the medical records. The duration of anesthesia was defined as the period from induction to emergence when SAD was removed, and the patients were ready for transfer to the ICU or PACU. Adverse respiratory events were defined as follows: apnea (assisted ventilation required > 10 seconds), desaturation (SpO2 < 95%), and laryngospasm. The baseline blood pressure measurements are usually unavailable for children because of poor cooperation, pain, anxiety, or other factors.3 Blood pressure below an age-specific range is considered more appropriate for the definition of hypotension during anesthesia for pediatric patients.4 Accordingly, hemodynamic instability was defined as hypotension (below two standard deviations from the age- and sex-specific reference ranges for systolic blood pressure in children during anesthesia)4 and bradycardia (decrease in heart rate > 25% from baseline). Each MRI scan was examined, and the image quality was judged by radiologists specialized in the interested areas observed through MRI. Data were presented as mean (standard deviation), median (interquartile range), or number (percentage, %) and were analyzed using Medcalc (MedCalc Software Bvba, Ostend, Belgium).
Results
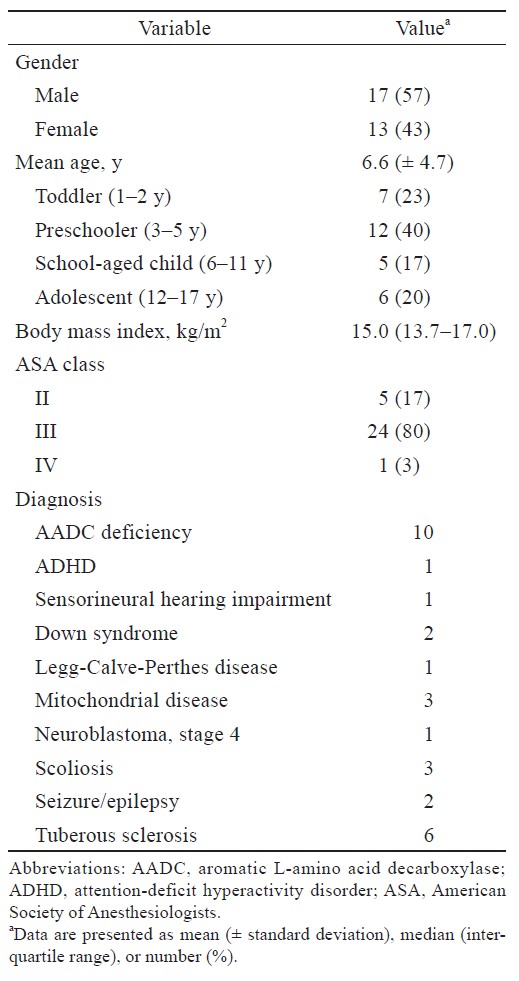
The demographic data and patient characteristics are presented in Table 1. There were 30 children (17 males and 13 females; median age 4 [interquartile range, 3.0–10.1; range, 1.6–17.0] years) with a total of 38 MRI exams. Most patients were considered to exhibit a high risk (III or IV) based on the American Society of Anesthesiologists physical status classification system (n = 24; 80%).
Download full-size image
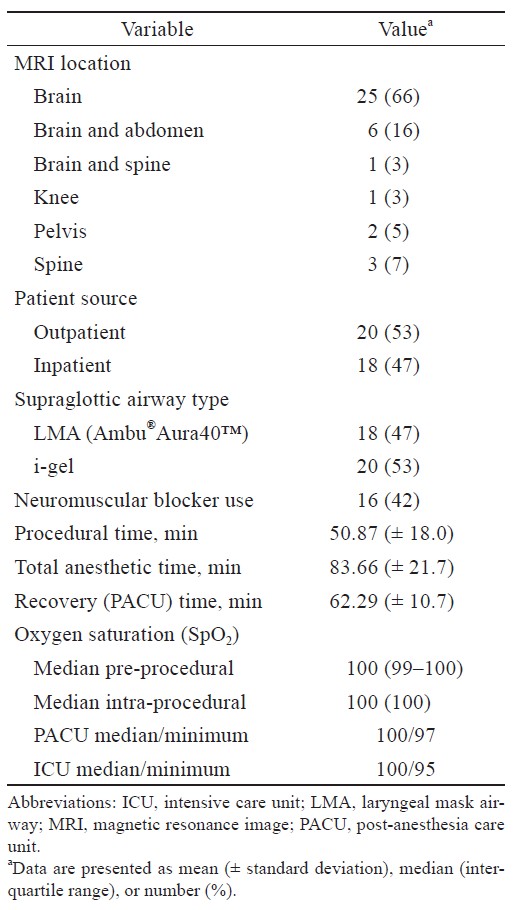
The anesthesia and MRI scan profiles are presented in Table 2. Among these 30 patients, 8 (5 with AADC deficiency and 3 with tuberous sclerosis) received two MRI exams (follow-up interval: 1 year) with a total of 38 MRI scans included and analyzed. The scans were taken in the brain (n = 25; 66%), brain/abdomen (n = 6; 16%), brain/spine (n = 1; 3%), knee (n = 1; 3%), pelvis (n = 2; 5%), and spine (n = 3; 7%). Eighteen scans were made using reusable Ambu® LMA (47%), and 20 scans were made using i-gel (53%). The mean duration of the procedure was 50.87 (± 18.0) mins. The mean total anesthesia time was 83.66 (± 21.7) mins, which was longer than the MRI exam duration because patients with AADC deficiency received lumbar puncture before the MRI exam. No respiratory adverse event was reported throughout the procedure for all patients. The procedure was successfully completed in all patients. Among the 38 MRI scans, three brain MRI images were reported with artifacts. One patient was reported to have moderate susceptibility artifacts because of the preexisting catheter of the ventriculoperitoneal shunt observed in one scan, and another patient was reported to have two mild motion artifacts (Ambu® LMA, i-gel) in two scans. However, the radiologist reported that the image quality of each scan was adequate for disease investigation.
Download full-size image
After the exam, all outpatients and inpatients, except patients with AADC deficiency, were transferred to the PACU before being discharged or transferred to the general ward. SADs were removed before each patient was transferred to the distant PACU or ICU. The mean duration in the PACU was 62.29 ± 10.7 mins. One 8-year-old child experienced a prolonged observation in the PACU (100 mins) because of transient bradycardia (heart rate, 55–65 rpm) but did not require treatment. No additional hemodynamic instability observed among other patients in the PACU. No patients experienced desaturation with supplemental oxygen needed, and no respiratory adverse event was reported in the PACU.
None of the patients with AADC deficiency experienced desaturation or respiratory adverse events in the ICU. Among the 10 patients with AADC deficiency, one 5-year-old boy experienced pre-anesthesia laryngomalacia, tracheomalacia, and a severe gastroesophageal reflux disorder. After SAD insertion, large amounts of gastric juice were noted on the gastric channel of the i-gel airway during the lumbar puncture. The problem was solved after gentle suction, the scan was completed successfully, and SAD was smoothly removed after the MRI scan. No respiratory complication was observed in the following hospital course.
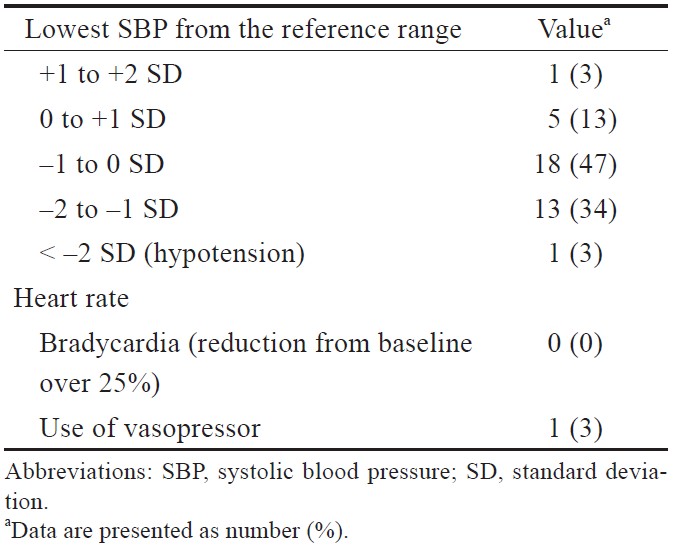
The hemodynamic profiles during the MRI are presented in Table 3. Hypotension was observed only in one 9-year-old patient (3%) with AADC deficiency near the end of the induction, and it was relieved by a single bolus dose of norepinephrine (2 mcg). During the MRI procedure, no patients experienced hypotension episodes or bradycardia.
Download full-size image
Discussion
In this study, we demonstrated that the use of SADs such as i-gel or Ambu® LMA for airway maintenance was reliable and safe for ill pediatric patients undergoing MRI scans.
The feasibility of using SAD for airway maintenance among children undergoing MRI under general anesthesia was reported in several studies that enrolled relatively healthy patients.5-7 Our study population predominantly comprised a high-risk population. Among these 30 patients, 10 patients had AADC deficiency, and 3 patients had mitochondrial disease. Therefore, MRI scans were particularly challenging because these patients were considered to have higher anesthesia-related risks.8,9 In addition, the literature on anesthesia outside the operating room for patients with AADC deficiency and mitochondrial diseases remains limited. Each of these 13 patients presented hypotonia, dystonia, hypokinesia at baseline, and were prone to have increased risks of anesthesia-related respiratory and cardiac depression. One of the advantages of using SADs for airway maintenance is that it reduces the requirement of anesthetics for induction and maintenance; in particular, it does not require a high dose of neuromuscular relaxant (< 0.15 mg/kg of cisatracurium for our patients). Therefore, it may prevent hemodynamic instabilities and respiratory complications for these high-risk patients. Although the anesthesia duration of the 10 patients with AADC deficiency was longer because of the additional lumbar puncture procedure, the respiration remained stable in the lateral decubitus position, and no respiratory adverse events due to prolonged anesthesia occurred in the ICU. In addition, only 1 patient (3%) experienced a hypotensive episode during anesthesia, and 1 patient (3%) experienced bradycardia in the PACU. Accordingly, our result revealed that for high-risk pediatric patients with, for example, AADC deficiency or mitochondrial diseases, using SADs for the maintenance of the airway is safe not only during MRI scans but also for special positionings, such as the lateral decubitus position for lumbar puncture. Notably, the use of SADs may also do high-risk pediatric patients good in terms of hemodynamic stability.
Conventionally, there is concern regarding the risk of aspiration for SAD use under general anesthesia. However, SADs are increasingly used in patients who are traditionally assumed to be at an increased risk for aspiration in recent decades. For instance, the SGA has been proposed to be an alternative to endotracheal intubation for laparoscopic surgery with comparable leak fraction even in the laparoscopic surgery in trendelenburg position.10,11 The choice of SAD with a gastric channel such as the i-gel may provide additional protection of aspiration because esophageal suction could be applied. For instance, one large case series including 280 patients reported that the use of i-gel protected from aspiration in 2 among 3 patients.12 In this case series, we observed that the gastric juice reflux was suctioned via the gastric channel of the i-gel without further complications in 1 patient with AADC.
The presence of a strong magnetic field in the MRI units has challenges for anesthetic care. The anesthetic equipment must be MRI-compatible, and the access to the patient is restricted. The i-gel airway has been reported to be a safe and useful device for MRI owing to its lack of ferromagnetic components, as demonstrated in an adult case series13 and a pediatric case series.14 However, i-gel was also reported to interfere with the proper positioning of the MRI head coils.15 We did not experience this condition in our patients. The i-gel airway was applied in 20 pediatric MRI scans, and each scan was completed successfully with adequate image quality including 14 brain area MRI scans (70%), 3 brain plus abdomen area scans (15%), and 3 other location scans (15%) in this case series. No adverse respiratory events were recorded during the transporting and positioning of children inside the MRI machine. We propose that i-gel is reliable and safe in pediatric MRI scans.
The image quality of MRI scans can be affected by various factors. Patient movement during the procedure not only generates artifacts that may interfere with the image interpretation but also interrupts the scan, which may imply repeating the procedure. Therefore, general anesthesia is considered to produce better image quality than sedation for high-risk children because of the avoidance of patient movement.2 For central nervous system MRI scans, the effects of anesthesia-related micromotion artifacts on image quality cannot be neglected. Recently, Ucisik-Keser and colleagues15 demonstrated that the use of an SAD was associated with the optimal brain MRI image quality in both pediatric and adult patients among four airway management strategies, namely no airway device, the use of an oral or a nasal airway, a SAD, and a tracheal tube. However, SADs may still lead to magnetic susceptibility artifacts because of ferromagnetic interference, which degrades the image quality and the diagnostic value. Zaballos et al.16 conducted an in vitro simulation study to investigate the artifacts created during MRI by six distinct types of SADs as follows: the classic LMA, LMA ProSeal, LMA Unique, Ambu® Disposable Pharyngeal Mask, LMA Supreme, and i-gel. This study revealed that, among the six SA the Ambu® Disposable Pharyngeal Mask and i-gel caused no artifacts because of the absence of metallic parts. In our clinical practice, either a reusable LMA (Ambu®Aura40™) or i-gel was chosen at the discretion of the attending anesthesiologist. The major difference between the Ambu® disposable and reusable LMA is that the second contains a ferromagnetic component in the pilot balloon. However, in our study, among the 15 scans using reusable Ambu® LMA, including brain area scans (brain only, n = 11; brain and abdomen, n = 3; brain and spine, n = 1), only one scan was reported to have mild motion MRI artifacts, and the image quality was deemed adequate by the neuro-specialized radiologist. Therefore, we consider that, with careful placement of the pilot balloon outside the MRI field, reusable Ambu® LMA is still an adequate airway alternative for pediatric central nervous system MRI scans.
The study is limited by its retrospective design. Therefore, minor adverse effects may be underestimated. For instance, although there were complete records of oxygen saturation in the anesthesia, PACU, and ICU notes, both apnea and laryngospasm may be under-measured based on medical records.
Conclusions
In conclusion, our study demonstrated that using both reusable Ambu® LMA and i-gel for airway maintenance during MRI is feasible and safe for pediatric patients including high-risk children, such as patients with AADC deficiency and mitochondrial diseases.
Conflict of Interest
The authors have no conflicts of interest to declare. The manuscript was presented at the European Anesthesiology Congress 2019.
Funding
This research was conducted without external funding.
References
| 1 |
Arthurs OJ, Sury M.
Anaesthesia or sedation for paediatric MRI: advantages and disadvantages.
Curr
Opin Anaesthesiol. 2013;26(4):489-494.
|
| 2 |
Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR.
Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes.
Br J Anaesth. 2000;84(6):743-748.
|
| 3 |
Nafiu OO, Voepel-Lewis T, Morris M, et al.
How do pediatric
anesthesiologists define intraoperative hypotension?
Paediatr Anaesth. 2009;19(11):1048-1053.
|
| 4 |
de Graaff JC, Pasma W, van Buuren S, et al.
Reference values for noninvasive blood pressure in children during anesthesia: a multicentered retrospective observational cohort study.
Anesthesiology. 2016;125(5):904-913.
|
| 5 |
Heard C, Harutunians M, Houck J, Joshi P, Johnson K, Lerman J.
Propofol anesthesia for children undergoing magnetic resonance imaging: a comparison with isoflurane, nitrous oxide, and a mask airway.
Anesth Analg. 2015;120(1):157-164.
|
| 6 |
Pedersen NA, Jensen AG, Kilmose L, Olsen KS.
Propofol-remifentanil or sevoflurane for children undergoing magnetic resonance imaging? A randomised study.
Acta Anaesthesiol Scand. 2013;57(8):988-995.
|
| 7 |
Bryan YF, Hoke LK, Taghon TA, et al.
A randomized trial comparing sevoflurane and propofol in children undergoing MRI scans.
Paediatr Anaesth. 2009;19(7):672-681.
|
| 8 |
Niezgoda J, Morgan PG.
Anesthetic considerations in patients with mitochondrial defects.
Paediatr Anaesth.
2013;23(9):785-793.
|
| 9 |
Vutskits L, Menache C, Manzano S, Haenggeli CA, Habre W.
Anesthesia management in a young child with aromatic l-amino acid decarboxylase deficiency.
Paediatr Anaesth. 2006;16(1):82-84.
|
| 10 |
Park SK, Ko G, Choi GJ, Ahn EJ, Kang H.
Comparison between supraglottic airway devices and endotracheal tubes in patients undergoing laparoscopic surgery: a systematic review and meta-analysis.
Medicine (Baltimore).
2016;95(33):e4598.
|
| 11 |
Lai CJ, Liu CM, Wu CY, Tsai FF, Tseng PH, Fan SZ.
I-Gel is a suitable alternative to endotracheal tubes in the laparoscopic pneumoperitoneum and trendelenburg position.
BMC Anesthesiol. 2017;17(1):3.
|
| 12 |
Gibbison B, Cook TM, Seller C.
Case series: protection from aspiration and failure of protection from aspiration with the i-gel airway.
Br J Anaesth. 2008;100(3):415-417.
|
| 13 |
Kaur K, Bhardwaj M, Kumar P, Lal J, Johar S, Hooda S.
Use of i-gel in magnetic resonance imaging.
Acta Anaesthesiol Taiwan. 2014;52(1):41-42.
|
| 14 |
Corso RM, Battelli D, Maitan S, Zampone S, Agnoletti V.
A clinical evaluation of the pediatric i-gelTM for airway management during MRI examination.
J Anaesthesiol
Clin Pharmacol. 2014;30(2):288-290.
|
| 15 |
Ucisik-Keser FE, Chi TL, Hamid Y, Dinh A, Chang E, Ferson DZ.
Impact of airway management strategies on magnetic resonance image quality.
Br J Anaesth. 2016;117(Suppl 1):i97-i102.
|
| 16 |
Zaballos M, Bastida E, del Castillo T, de Villoria JG, Jiménez C.
In vitro study of magnetic resonance imaging artefacts of six supraglottic airway devices.
Anaesthesia. 2010;65(6):569-572.
|