Abstract
There have been immense advances in the safety and variety of intravenous anesthetic delivery systems including drug cost reduction, development of more effective opioids, and improvement in depth of anesthesia monitoring in the last 20 years. Propofol-based total intravenous anesthesia (TIVA) with target-controlled infusion (TCI) is relatively easy to practice. While this technique promotes a higher overall anesthesia quality and patient survival, especially for cancer patients, there are deficiencies in training and education of the technique. Therefore, the Society for Intravenous Anesthesia and the Association of Anesthetists (United Kingdom) have laid out guidelines in an attempt to highlight multiple important TIVA-related safety issues to help clinicians feel more confi dent. In the present article, we discuss fi ve recommendations and four special clinical situations. Preparation, equipment familiarity, and safe delivery techniques are extremely important for the proper employment of this method. Herein, we emphasize the importance of proper education, and the clinical practice experience of the TIVA technique. Additionally, we suggest a modifi ed connection method to set up a safely administered line. We highlight the advantages of using processed electroencephalogram monitoring (such as bispectral index or Entropy) to prevent awareness during TIVA administration in difficult clinical situations. These situations may include triple low patients (e.g., low blood pressure, low maintained effect-site concentration of propofol, and low body weight ≤ 18), obese patients, and patients with diffi cult infusion site monitoring or use of neuromuscular blocking agents. Due to a limited consensus among Taiwanese medical professionals, this document is intended to act as a safe practice reference for the use of TIVA with TCI. Additionally, two pithy formula codes, 4321 for propofol with fentanyl/alfentanil and 42222111 for propofol with remifentanil, are provided for the general population and one pithy formula code, 4321 for propofol with fentanyl, is provided for pediatric patients.
Keywords
propofol, recommendation, remifentanil, targetcontrolled infusion (TCI), total intravenous anesthesia (TIVA)
Introduction
In 2005, a target-controlled infusion (TCI) system with the software designed pharmacokinetic (PK) model that can estimate drug levels in the blood and brain, was first imported into Taiwan. This model allowed for automatic modification of the drug infusion rate to achieve a target concentration. In the same year, the Department of Anesthesiology at Tri-Service General Hospital (TSGH) started the clinical application of combined total intravenous anesthesia (TIVA) with TCI. In comparison with the most commonly used technique worldwide, inhalation anesthesia (INHA), TIVA with TCI has proven to be more environmentally friendly with reduced greenhouse effects. This technique not only reduces recovery and extubation time but also yields a higher standard of anesthesia quality. As a result of these advancements, the Department of Anesthesiology at TSGH received two Symbol of National Quality (SNQ) awards, in 2007 and 2015, due to the successful application of this technique.
To assure the safety and high quality of TIVA with TCI in clinical settings, we have integrated previous user experience, along with common questions and answers, and various surgical examples into a TCI Manual (two versions in Chinese and one in English). The goal was to produce a manual to be used as a standard handbook for clinical practice and education that serve to expedite the transfer of knowledge of this technique to anesthetists or other people that are interested and thereby benefit a wider population of surgical patients. To this end, we have hosted TIVA with TCI training courses since 2009. In conjunction with clinical observations and simulation-based education, our TCI Manual was used as a reference handbook to actively promote the technique nationwide. As a result of our efforts, we received perfect scores from a satisfaction survey targeting at trained anesthetists and received another SNQ award in 2019. Propofol-based TIVA has shown to reduce costs, the risk of postoperative nausea and vomiting (PONV), and emergence latency for several surgeries,1-9 except for open liver resection in Taiwan.10 Quality of recovery is one of the most obvious, well-known, and tangible benefits of propofol-based TIVA, but others are becoming more apparent.5,11 Patients under propofol- based TIVA appear to experience less pain than those under INHA.12,13 Additionally, propofol-based TIVA is associated with less severe effects on neurocognitive function.14 Furthermore, relative to INHA, propofol-based TIVA is associated with higher survival rates in many cancer surgeries,15-21 excluding breast cancer.22 These advantages, however, still appear to be insufficient to persuade many anesthesiologists to adopt the use of TIVA for their patients.23
More than 20 years after the introduction of propofol-based TIVA, there is an apparent deficiency in quality of care when anesthesiologists use this technique, as the 5th National Audit Project (NAP5)23 reported that TIVA was correlated with a disproportionate number of accidental awareness cases under general anesthesia (GA). If familiarity breeds contempt, it is unlikely to be the case with propofol-based TIVA as it accounts for only 8% of GA.23 However, at TSGH, we have a very low incidence of accidental awareness, only 0.017%, due to our well-trained staff and strict protocol for standard practice that is renewed yearly.24 Therefore, we propose that the establishment of a well-defined and standard practice protocol that is renewed yearly is highly suggested for the effective use of TIVA.24
Inadequate education and training are still the main factors of TIVA-related accidental awareness under GA.23 Making mistakes during TIVA use may result in failed drug delivery, wrong dosage, wrong concentration, or other problems. In the NAP5, the two most common causes of intraoperative awareness under TIVA are failed delivery of an intended drug dose and poor understanding of underlying pharmacological principles.23 It is critical that anesthetists are properly trained to be competent in the delivery of TIVA because we may face some circumstances that impede the use of a volatile anesthetic (VA). To ensure proper use, hospitals must be required to offer education and the clinical practice experience of TIVA administration to every anesthesia staff. For hospital that possesses inadequate TIVA training, TSGH can provide these services.
Since well-defined recommendations and guidelines of TIVA-TCI are absent in Taiwan, we would like to share our experiences to ensure the safe practice of TIVA. In the present article, we discuss the following five recommendations: (1) continuing education and competency in TIVA, (2) achieving the desired drug concentration, (3) selecting a proper target drug concentration, (4) familiarizing with the principles of the safe practice of TIVA with TCI, and (5) monitoring a patient during TIVA; the four special clinical situations discussed are as follows: (1) propofol-related infusion syndrome, (2) TIVA in rapid sequence induction (RSI), (3) TIVA in pediatric practice, and (4) sevoflurane as an adjuvant for TIVA in particular circumstances.
Recommendations
Continuing Education and Competency in TIVA
Every anesthesia staff, including anesthesiologists and anesthetic nurses, should be trained and competent in the delivery or practice of TIVA. Hospitals should provide teaching, training, and practical experience of TIVA to all anesthesia staff. Attending physicians should take responsibility to make sure that all applicable staff get know-how for the safe delivery of TIVA.25
When TIVA was initially used in a clinical setting, drug delivery, as quantified by manual weightbased algorithms (10-8-6 mg/kg/hr), was cumbersome and often inexact regarding to dosing and prolonged infusion. Moreover, there was a limited array of affordable and potent short-acting opioids, resulting in excessive costs associated with anesthetic use. However, over the last 20 years, delivery systems have become safer and more widely accessible, drug costs have plunged, more effective opioids, such as alfentanil and remifentanil, have been developed, and the depth of anesthesia monitoring has become widely available. The introduction of TCI with PK modeling made this much easier. As a result of these developments, TIVA with TCI has become an efficient and safe technique.26 While some case reports have stated that TIVA is more susceptible to human error than INHA, including errors associated with the set-up of IV infusions, no reports have suggested that a TCI mode of drug delivery introduces unique safety issues.27 To counteract deficiencies in TIVA related education and training, Nimmo et al.25 from the Society for Intravenous Anesthesia and the Association of Anesthetists outlines a number of important safety issues and promotes confidence among clinicians to properly utilize the technique.
The safe and effective delivery of TIVA is critical for situations when the administration of INHA is not possible. Hospitals or medical schools should offer proper education and clinical practice experience of TIVA to every anesthesia staff. Therefore, training in the administration of TIVA must be required as one of the core programs in anesthesia education. Anesthetic trainees must be able to conduct TIVA before being left unsupervised to take care of patients undergoing TIVA. The Department of Anesthesiology at TSGH has provided a TCI training course for many years and thus received an SNQ award in 2019.
Achieving the Desired Drug Concentration
(1) A TCI is suggested while anesthetists use propofol infusion for maintenance of GA. The user selects the drug and PK model to be used by that TCI pump and inputs the patient characteristics (covariates), such as body weight and age, and target plasma (Cp) or effect-site concentration (Ce), with the pump programming the initial bolus and subsequent infusion rates. Marsh and Schnider models are the two most widely used adult propofol models.25
(2) All anesthesia staffs need to know the PK and pharmacodynamics of IV anesthetics or analgesics to be competent in choosing proper Cp or Ce for TIVA drugs for the case. Achieving a stable Cp or Ce of a drug needs varying drug infusion rates and time. For instance, during induction and maintenance of anesthesia, boluses, or rapid infusion should be followed by reducing the infusion rate and the targeted anesthetic Cp or Ce could be predicted from the PK models.25 Thus, manual boluses, infusion pumps, or TCI pumps could be used for induction and/or maintenance of anesthesia. The same PK model may be proper for cases with similar characteristics (e.g., young, healthy, and not morbidly obese).28 Conversely, caution is needed while applying models in cases with dissimilar characteristics (e.g., ASA classification 3–5, elder, morbidly obese, and hypovolemic cases). The Schnider and Marsh models are the most widely used models to robust adults25; the Kataria29 and Paedfusor30 models apply specifically to children. Propofol Cp in individual cases may not be the same between the value estimated by the PK model and shown by TCI pumps. The mean difference between predicted and measured concentrations is < 25%.31 However, if Cp of the cases differs from Cp of the population in which the model was established, the difference may be theoretically greater. In these situations, TCI pumps may be helpful devices for titrating propofol infusion to effects of anesthesia (e.g., clinical effects or the processed electroencephalogram [pEEG] monitor), but the estimated propofol concentration should be not precise.
(3) The Association of Anesthetists and the Society for Obesity and Bariatric Anesthesia have reported the guideline including the deliberation on the use of TIVA in morbidly obese patients.32 There is no consensus about whether total body weight or another scalar, such as adjusted body weight, is more effective in calculating proper doses for TCI pump usage in obese patients. Moreover, the Marsh and Schnider PK models and the estimated propofol Cp or Ce may be inexact in the morbidly obese cases. The maximum body-weight accepted by the Marsh TCI pump is 150 kg, and the pump by the Schnider model only accepts variables that include a body mass index (BMI) less than 35 kg/m2 for female or less than 42 kg/m2 for male. While one conducts TIVA with TCI in the morbidly obese cases, it is recommended to use lean body mass or adjust height to match the chosen PK model and then titration by clinical effect and pEEG monitoring.24,25
Selecting a Proper Target Drug Concentration
(1) The target concentrations of anesthetics should be selected based on the patient characteristics, co-administered drugs, and clinical conditions:
Target combinatorial concentrations of propofol and analgesic should achieve loss of consciousness and avoid movements while noxious stimuli. However, excessively high concentrations should not be given to avoid hypotension and delayed emergence from anesthesia. Importantly, there are no fixed propofol or analgesic Cp or Ce that are proper for all patients. Rather, the determination of IV anesthetic concentrations depends on individual case variation, co-administered anesthetics, and the surgical stimulus level. While the required propofol Ce could not be estimated, observations of response of cases during anesthesia induction might help approximate the propofol concentration required for maintenance. If a rapid induction of anesthesia is needed, an initial propofol Cp (Marsh model) or Ce (Schnider model) of 4.0–6.0 μg/mL is commonly administrated in robust young or middle-aged cases. Generally, younger cases need a higher Ce of anesthetics than elder cases.25 Alternatively, slower anesthesia induction requires setting a lower initial target propofol concentration (e.g., 1.0 μg/mL), followed by repeated 0.2–0.5 μg/mL incremental step increasing in the target concentration. This modality should be helpful for elder or weak cases because it is related to less marked hypotension after induction. In addition, a slower induction enables anesthetists to identify the estimated Ce at which cases lose consciousness. During maintenance of anesthesia, the target concentration of propofol and opioid administration should be adjusted by the clinical signs. If patients receive neuromuscular blocking drugs (NMBDs), maintenance of anesthesia should be monitored with pEEG devices.25 During maintenance of anesthesia, target concentrations of 3.0–6.0 μg/ mL (without opioids) or 2.0–4.0 μg/mL (with opioids) are argued and an adjustment of anesthetic depth with repeated 0.2–0.5 μg/mL increasing or decreasing propofol is suggested. Higher initial target drug concentrations may be necessary for healthy or agitated cases, whereas lower target drug concentrations are more proper for elder and frail cases.25 In addition, use of benzodiazepines, ketamine, a2-adrenoceptor agonists, magnesium, and nitrous oxide may reduce the required propofol Ce.25 Moreover, the regional anesthetic block may decrease required propofol concentrations. Coadministration of opioids reduces the propofol consumption to provide a loss of consciousness and blunt movement and hemodynamic responses to noxious stimuli.33 Remifentanil infusion is often used in conjunction with propofol TCI to create a highly effective anesthesia.25 In the case of remifentanil TCI co-administered with propofol, target remifentanil concentrations of 2.0–4.0 ng/mL are usually chosen and may necessitate lung ventilation because spontaneous breathing is unusual while concentration is higher than 1.5 ng/mL in adults. The rapid offset of effects after the termination of remifentanil use enables the administration of doses to decrease propofol consumption about 50% without inducing lengthy respiratory suppression following procedures.25 Nevertheless, intraoperative remifentanil does not cover postoperative analgesia. Moreover, higher remifentanil dosage (Ce > 5.0 ng/mL or infusions > 0.2 μg/kg/min) may induce acute opioid tolerance or remifentanil-induced hyperalgesia.34 Thus, preventing remifentanil-induced hyperalgesia is critically important.35,36 The closure of subcutaneous tissue during wound closure may allow for the adjustment of propofol Ce to 2.0 ug/mL, especially for prolonged anesthesia to facilitate emergence from anesthesia. Additionally, awake propofol Ce should be estimated via the following formula: 1.66 − (age × 0.01). We also suggest to gradually decrease the remifentanil infusion Ce to 1.0 ng/mL until extubation to prevent remifentanil-induced hyperalgesia and unstable hemodynamics during emergence.36
(2) Two pithy formula codes, 4321 for propofol with fentanyl/alfentanil and 42222111 propofol with remifentanil, are provided in Table 1 for the general population. Additionally, preventing remifentanil- induced hyperalgesia is suggested.35,36
Download full-size image
Principles of the Safe Practice of TIVA With TCI
(1) One concentration of a drug:
The typical protocol in the department of anesthesia is to use only one concentration of propofol or dilute remifentanil (50 μg/mL), or alfentanil (100 μg/mL).25 If there are two or more drug concentrations, it is suggested to set up a protocol to minimize the risk of drug error.
(2) Sterile technique:
The rubber stopper or ampule neck of the propofol should be sanitized by rubbing alcohol before use. Additionally, the new sterile syringe and drawing up device should be applied each time to decrease the incidence of contamination. Every syringe should be prepared as shortly as possible before administration, and those not presently applied should be sealed.
(3) Choose the right PK model and brand of syringe:
When one controls the TCI pump, variables should be strictly defined drug concentrations, PK model(s), and the type of syringe to decrease variable associated risks. It is important to note that syringes with the same size from several manufacturers have varying internal diameters. Therefore, the infusion pump must be programmed according to the brand of syringe.25 However, not all TCI pumps allow for the selection of syringe type. Therefore, a single syringe brand should be used within a department. If the same syringe brand is used in a department, variations in TCI delivered volumes could be omitted.
(4) The safe administered set:
The infusion sets for TIVA delivery should be equipped with the Luer-lock connector at each end to decrease the risk of incidental disconnection. Also, the infusion set should contain an anti-siphon valve on the drug delivery line(s) to prevent uncontrolled infusion from a damaged syringe or backward drug flow, especially while the pump is beneath the infusion site. If there are two-time or more infusion of drugs administrated via a single IV cannula, an anti-reflux valve should be used to avoid backward drug flow in the infusion tube.
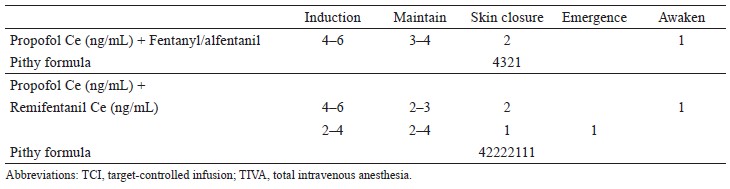
We suggest using a BD Q-syte, which is composed of a Luer-lock connector and an anti-siphon valve, connected to an IV catheter (Figure 1A, black arrow) and infusion set (Figure 1A, red arrow), allowing for the delivery of TIVA. Another BD Q-syte, the anti-siphon valve is connected to the syringe (Figure 1B, red arrow), and the Luer-lock connector is connected to the the drug delivery line (Figure 1B, black arrow).
Download full-size image
(A) A BD Q-syteTM, with the Luer-lock connector (black arrow) and the anti-siphon valve (red arrow) connceted to intravenous (IV) catheter and infusion set, respectively. A low priming volume infusion line for drug delivery, and it was labelled (white arrow, PAHSCO Pressure Tube, 150 cm in length and a volume of 0.9 mL). The IV line connected to three-way stopcock sholud be taped with 3M tape (orange arrow). (B) TCI infusion pumps were programmed after the syringes containing the drugs to be infused have been placed in the pumps. We suggest that the fi rst, second, and third-layer pump sholud infuse propofol, opioids, and neuromuscular blocking drugs, respectively. Using a BD Q-syteTM, the anti-siphon valve connceted to syringe (red arrow) and the Luer-lock connector connected to the drug delivery line (black arrow). (C) Use a infusion line, 90 cm in length and a volume of 0.9 mL, while wrapping a hand (yellow arrow, JSM Pressure Tubing).
Drug and fluid lines should be linked together as close to the case as possible to minimize dead space (Figure 1A).25 Figure 1 shows that the low priming volume infusion lines used for drug delivery will reduce excessive drug waste after surgery and limit the impact of possible free flow (white arrow, 150 cm in length and a volume of 0.9 mL; yellow arrow, 90 cm in length and a volume of 0.9 mL). In addition, these lines allow for delayed drug delivery to be minimized upon the termination of IV dripping. Moreover, upon the restarting of IV dripping, the volume of drugs flushed into the patient is negligible. Accordingly, this can greatly reduce risk of drug overdose or awareness.
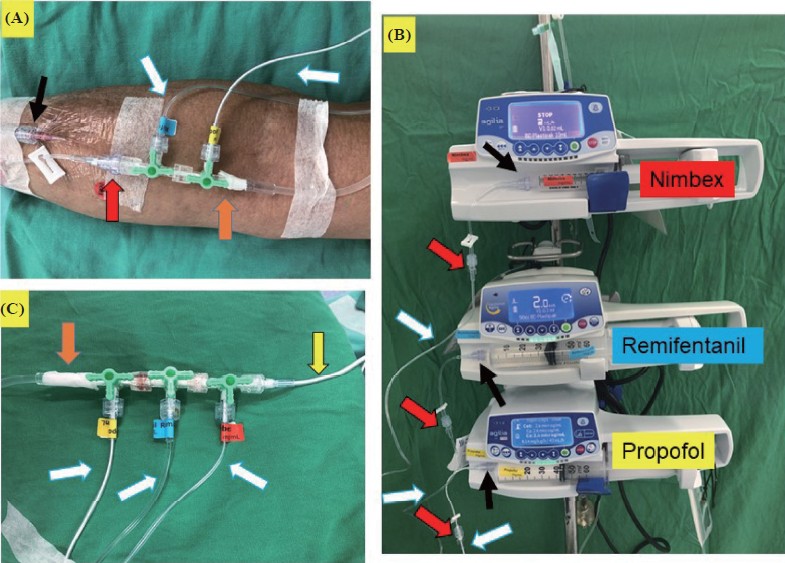
Each infusion line delivered each drug and labeled is recommended (Figure 1A–C, white arrow). The IV line connected to the three-way stopcock should be taped with 3M tape to prevent accidental disconnection (Figure 1A and C, orange arrow). Furthermore, administration sets should be specifically designed for TIVA (Figure 2).25 As much as possible, it is critical to minimize potential leakage or disconnections sites for TIVA infusion lines by self-assembly. As an ideal infusion line, there are no additional connections or three-way taps from the syringe to the cannula. Specifically, designed TIVA administration sets (Figure 2) are chosen in order to satisfy above requirements, which are better than self-assembled sets. Unfortunately, designed TIVA administration sets are unavailable in Taiwan. Therefore, Figures 1 and 3 outline the most effective procedure for modifying drug infusion line connections to avoid issues discussed above. We also suggest using ≤ 3 three-way stopcocks for TIVA administration (Figure 1C).37
Download full-size image
The administration sets with luer-lock connectors, anti-siphon and anti-refl ux valves is specifically designed for TIVA such as (A) Fresenius TIVA set infusion system with 2 three-way taps and (B) venous infusion set, TIVA set, Mediplus, United Kingdom.
(5) Infusion pumps should only be programmed after the syringe containing the infused drug has been placed in the pump.25 In addition, the first, second, and third-layer pump should infuse propofol, opioids, and NMBD, respectively (Figure 1B).
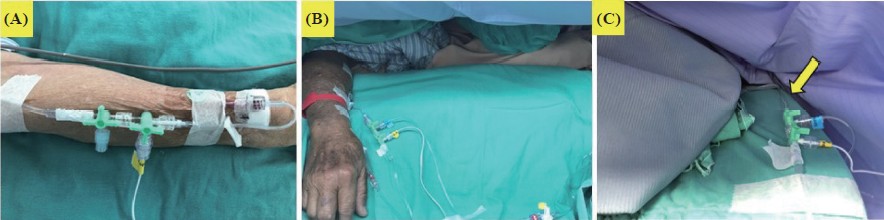
(6) The IV cannula or central venous catheter through which the infusion is being delivered should be visible during anesthesia as much as possible (Figure 3):25
Download full-size image
(A) holding out a hand on the hand stand of the operating table in the supine position, (B) holding out a hand in the prone position, and (C) wrapping a hand in the supine position with a infusion line (yellow arrow, 90 cm in length and a volume of 0.9 mL).
Intraoperative awareness in cases under TIVA usually results from the IV cannula related failed drug delivery. Thus, previous reports have recommended the constant visibility of the TIVA delivering IV cannula.25 However, uninterrupted observation of the IV cannula may not be executed during some operations. In such situations, anesthetists should periodically inspect infusion problems and check for the cannula site. The threshold for applying pEEG should be decreased in such situations. If the patient’s response to the infused anesthetic(s) reveals unusual, the cannula site should be fi rst checked.
(7) Functional alarm system:
Both TCI infusion and fixed-rate infusion pumps should be equipped with audible alarms. Alarms should be activated by high pressure, stopped infusion, empty syringe, main electricity supply disconnection, and low battery. In addition, pumps with alarms for drops in pressure may detect some disconnections. Moreover, pumps should include a visual display to reveal infusion progress throughout anesthesia.
(8) Pumps should be charged before use and be powered during use to avoid device failure from unexpected battery depletion:
If the TCI pump shuts down by unexpected battery depletion, or be rebooted due to the malfunction, the restart of TCI anesthesia using the previous target concentration is not appropriate during anesthesia. In the event TCI is restarted, the pump would not account for any previously administered drug concentrations, thereby leading to another rapid infusion induction dose with an overly higher drug concentration. The following modality has been suggested upon pump shut down: [1] bolus infusion of propofol 20–50 mg, [2] open a VA 0.2–0.5 minimal alveolar concentration, [3] restart the (or a new) TCI pump with half of the Ce at the time of failure, and [4] adjust the Ce of anesthetics according to the pEEG and hemodynamics.
(9) Before TIVA administration, double-check the drugs to be administered and confirm the programming of the pump, the infusion set, and the IV cannula:
Upon TIVA induction, it is important to periodically verify the dose of anesthetics given via the pump. If required, an NMBD should only be given after a confi rmed loss of consciousness. While one uses manual bolus, the displayed drug concentrations may be inexact for few minutes. As stated above, the infusion pump should be visible during anesthesia. Moreover, every few minutes, anesthetists should check the infusion rate.
Infusion pumps should be programmed following the placement of the drug-containing syringe. Intraoperative awareness may happen while one places propofol syringe in the preprogrammed pump.25 These mistakes may be improved by using a consistent layout of pumps, such as setting the propofol infusion pump at the first layer of any pumps. Drug name and concentration should always be labeled on syringes (Figure 1B). Finally, drug labels should be attached to syringes only while anesthetists draw up the intended drug.25
(10) Avoid residual anesthetics after surgery:
In TIVA, the vascular access devices should be flushed with more than two times the dead space volume of the devices, followed by removal of the infusion tube and the three-way stopcock at the end of surgery. If these procedures are not completed, potent anesthetic drugs (e.g., remifentanil or NMBDs) can remain in the vascular access device dead space and be accidentally given to the case postoperatively.25
Monitoring the Patient During TIVA
(1) While anesthesiologists use NMBDs with TIVA, applying the pEEG monitor is suggested:25
Anesthesiologists should be proficient in the principles, interpretation, and limitations of pEEG monitoring.25
Conducting TIVA needs an individualized analysis of the risks (e.g., awareness) and benefits (e.g., less PONV, agitation, postoperative pain, and better outcomes) for each patient. Previous studies have found the relationship between TIVA and higher risk of intraoperative awareness during GA,23 whereas others have not.24 More importantly, multiple observational studies found that TIVA was more significantly associated with improved outcomes after cancer surgeries than INHA15-21,38 but the results of randomized trials are still not available. Therefore, it is important for anesthetists to provide patients with adequate information regarding available techniques. However, it may be unwanted and unrealistic to explain the type of anesthesia in the consulting process in detail.39
If required, an NMBD should only be given after the confirmed loss of consciousness. In addition, applying the pEEG monitor is suggested while an NMBD is used with TIVA. The majority of intraoperative awareness cases identified in NAP5 happened in cases with NMBDs.22 Monitoring with pEEG should be commenced before the administration of an NMBD. Cases of awareness reported in the NAP5 commonly happened around the time of anesthesia induction and transfer from the anesthetic induction room to the operating room (OR). During the INHA maintenance phase, it is feasible to use the end-tidal VA concentration as an indication that the VA is being delivered as required; however, this is not possible under TIVA until now. Therefore, monitoring the depth of anesthesia with a pEEG monitor may decrease the incidence of awareness.40 About 20% of the NAP5 reports of awareness happened at the end of procedures due to the residual NMBD effect when the patient regained consciousness.23 Keeping the propofol Ce around 1.0–1.5 ug/mL is suggested in the absence of spontaneous breathing. Finally, strategies for preventing awareness are using pEEG monitoring in high-risk surgical patients during TIVA, such as triple low patients (e.g., low blood pressure, low maintained propofol Ce, and low body weight (BMI ≤ 18), previous history of alcoholism, previous history of awareness, obese patients (BMI ≥ 35 kg/m2 in female and ≥ 42 kg/m2 in male), poor functional activity (< 4 metabolic equivalents or ejection function < 35%), and as per patients’ request. Alternatively, 2.5–5.0 mg of midazolam has been administered for the prevention of intraoperative awareness in cases that require higher propofol Ce (> 6.0 μg/mL).24
(2) While TIVA is applied outside OR, the same standards of practice and monitoring should also be used for anesthesia in the OR.25
Special Clinical Situations in TIVA
(1) Propofol-related infusion syndrome:
Propofol-related infusion syndrome is a rare complication with potentially fatal results. Interruption in mitochondrial energy production results in rhabdomyolysis, acidemia, and multi-organ failure. Prolonged infusion (> 48 h), high propofol delivery rate (> 5.0 mg/kg/h), critical illness, low intake of sugar, and co-administration of catecholamines or steroids are risk factors.41 Some uncommon metabolic disorders, such as mitochondrial disease, fatty acid oxidation disorders, and co-enzyme Q deficiency, may increase the risk of developing propofol-related infusion syndrome.
(2) RSI and TIVA:
In RSI, TCI of propofol should be performed by setting an initial higher target concentration, thereby delivering the induction “bolus” dose of propofol as a rapid infusion (e.g., 1,200 mL/h). Once the intended dose has been given, the rapid infusion is then followed by a reduction to the target concentration. While using the TCI propofol pump for RSI, the induction dose of propofol is commonly given slower than a manual bolus dose. The time to loss of consciousness may be decreased by coadministration of other fast onset drugs, including remifentanil or alfentanil. While the propofol bolus is given manually rather than by the TCI pump in induction of anesthesia, the propofol Cp revealed by the pump may be inexact in the early period of anesthesia. An alternative approach is the administration of a bolus of a different drug, such as thiopentone or etomidate, for the RSI, followed by a TCI of propofol for maintenance of anesthesia. Paradoxical increases in pEEG index value may happen while using ketamine.42
(3) TIVA in pediatric practice:
Anesthetists providing TIVA to children need specific knowledge to equate for the pharmacological and clinically practical differences in this age range. The Kataria and Paedfusor models are the two commonly used pediatric TCI programs (target Ce or Cp of propofol).25 The Kataria model can be used in children aged 3–16 years and weighing 15–61 kg. Conversely, the Paedfusor should be used in children aged 1–16 years and weighing 5–61 kg. Teenage children weighing more than 61 kg should be conducted by the Marsh adult model.25
Pain is typically observed during induction of anesthesia and may be alleviated with pre-administration of IV opioids or lidocaine. While one switches to TIVA following induction with sevoflurane, it is crucial to prevent an improper propofol Ce. It may be accomplished by using an initial propofol target of 4.0 μg/mL and reducing the target after the pump reveals that a 2.0–3.0 mg/kg bolus has been administrated or set Cp 3.0 ug/mL after anesthetists stop sevoflurane. While one administrates an analgesic adjunct including remifentanil or the regional blockade, the target concentration of propofol during maintenance of anesthesia may be decreased about 50%.25 It is very crucial that the target concentration of 5.0–6.0 μg/ mL for children aged less than 12 years will lead to propofol accumulation, inducing delayed emergence after anesthesia. When an opioid is co-administered, a target concentration of 2.5–4.0 μg/mL for propofol is usually proper during the maintenance of anesthesia. However, in certain situations, the intended target concentrations may be out of this range. In these cases, it is necessary to titrate the dose to effect and utilize sound clinical judgement. Herein, we also provide the pithy formula code 4321 for propofol with fentanyl in pediatric patients.
Children aged less than 8 years are inclined to be less sensitive to the effects of remifentanil. This age group typically tolerates higher doses while spontaneous breathing and requires larger doses to induce antinociceptive effects.43,44 TCI of remifentanil can be used for adult target concentrations and the Minto model for cases aged less than 12 years and weighing more than or equal to 30 kg. For younger children, it is needed to administrate a manual infusion, as lower doses, such as 0.1–0.2 μg/kg/min, are not yet available for TCI.
In children, monitoring via pEEG should be applied to guide TIVA use. Nevertheless, the effects of anesthetics on EEG recordings in children under the age of 1 year differ from those in older children and adults.45 Importantly, applying pEEG monitors is suggested while using NMBD in children aged more than 1 year.
(4) Using sevoflurane as an adjuvant for TIVA in particular circumstances:
During laryngeal surgery, secretions are more common while under propofol-based TIVA than INHA.46 In addition, ophthalmologists have concerns regarding the relationship of postoperative endophthalmitis and TIVA, while excess nasal secretions flowing into the ophthalmic operative field might interrupt the surgical procedure during TIVA.47 However, in our previous study, we did not find the correlation between postoperative endophthalmitis and TIVA.48 To address excess secretions, a combination with 1% sevoflurane anesthesia has been used to attenuate secretions under propofol-based TIVA.49 Moreover, this combination does not increase the incidence of PONV or prolong extubation in ocular surgery.49
Recently, innovations in using TIVA for non-intubated video-assisted thoracic surgery have been further used. A cough reflex is an inevitable problem during surgeries that require approaching central lung lesions and that may induce major bleeding. Combination with 1% sevoflurane anesthesia may attenuate cough reflex under propofol-based TIVA without increasing the risk of PONV in non-intubated video- assisted thoracic surgery with laryngeal mask airway and spontaneous breathing without vagus nerve blockade.50
Conclusions
Until now, complete adherence to a central TIVA guideline remains elusive due to the unavailability of administration sets or anti-reflux valves in Taiwan. Considering the limitations associated with the use of TIVA in clinical practice, herein we have made several recommendations for the safe practice of TIVA. We suggest that the availability of educational resources covering the administration of TIVA and concomitant EEG monitoring need to be expanded to ensure the safe use of this technique.
In addition, to properly and safely use TIVA with TCI, anesthetists must be required to know the following: (1) the principles of inducing and maintaining proper Cp and Ce of the IV anesthetics; (2) the crucial factors for proper target drug concentrations and how to adjust concentrations in response to patient variability; (3) making sure that the required drug dose is given to the case; (4) applying pEEG monitors for cases undergoing TIVA with TCI.
Author Contributions
Hou-Chuan Lai conducted the study and wrote the main manuscript text. Yi-Hsuan Huang helped data collection and prepared the main manuscript text. Jen-Yin Chen helped data collection, and prepared the main manuscript text. Chih-Shung Wong helped data collection and prepared the main manuscript text. Kuang-I Cheng helped data collection and prepared the main manuscript text. Ching-Hui Shen helped data collection and prepared the main manuscript text. Zhi-Fu Wu designed the study and wrote the main manuscript text.
Conflict of Interest
The authors declare no competing interests.
Funding
None.
References
| 1 |
Chan WH, Lee MS, Lin C, et al.
Comparison of anesthesia-
controlled operating room time between propofol-
based total intravenous anesthesia and desflurane anesthesia in open colorectal surgery: a retrospective study.
PLoS One. 2016;11(10):e0165407.
|
| 2 |
Lai HC, Chan SM, Lu CH, Wong CS, Cherng CH, Wu
ZF.
Planning for operating room efficiency and faster
anesthesia wake-up time in open major upper abdominal
surgery.
Medicine (Baltimore). 2017;96(7):e6148.
|
| 3 |
Lai HC, Hung NK, Lin BF, Chen JL, Huang YH, Wu
ZF.
Lower incidence of prolonged extubation in propofol-
based total intravenous anesthesia compared with
desflurane anesthesia in laparoscopic cholecystectomy:
a retrospective study.
J Med Sci. 2019;39(3):121-126.
|
| 4 |
Wu ZF, Jian GS, Lee MS, et al.
An analysis of anesthesia-
controlled operating room time after propofol-based
total intravenous anesthesia compared with desflurane
anesthesia in ophthalmic surgery: a retrospective study.
Anesth Analg. 2014;119(6):1393-1406.
|
| 5 |
Lu CH, Wu ZF, Lin BF, et al.
Faster extubation time with
more stable hemodynamics during extubation and shorter
total surgical suite time after propofol-based total intravenous
anesthesia compared with desflurane anesthesia
in lengthy lumbar spine surgery.
J Neurosurg Spine.
2016;24(2):268-274.
|
| 6 |
Lai HC, Chan SM, Lin BF, Lin TC, Huang GS, Wu ZF.
Analysis of anesthesia-controlled operating room time after
propofol-based total intravenous anesthesia compared
with desflurane anesthesia in gynecologic laparoscopic
surgery: a retrospective study.
J Med Sci. 2015;35(4):157-
161.
|
| 7 |
Liu TC, Lai HC, Lu CH, et al.
Analysis of anesthesia-
controlled operating room time after propofol-based
total intravenous anesthesia compared with desflurane
anesthesia in functional endoscopic sinus surgery.
Medicine (Baltimore). 2018;97(5):e9805.
|
| 8 |
Horng HC, Kuo CP, Ho CC, et al.
Cost analysis of three
anesthetic regimens under auditory evoked potentials
monitoring in gynecologic laparoscopic surgery.
Acta Anaesthesiol
Taiwan. 2007;45(4):205-210.
|
| 9 |
Chan SM, Horng HC, Huang ST, Ma HI, et al.
Drug cost
analysis of three anesthetic regimens in prolonged lumbar
spinal surgery.
J Med Sci. 2009;29(2):75-80.
|
| 10 |
Lai HC, Huang YH, Lu CH, Hung NK, Wong CS, Wu ZF.
Comparison of anesthesia-controlled operating room
time between propofol-based total intravenous anesthesia
and desflurane anesthesia in open liver resection: a retrospective
study.
Asian J Anesthesiol. 2020;58(2):64-71.
|
| 11 |
Lu CH, Yeh CC, Huang YS, et al.
Hemodynamic and biochemical
changes in liver transplantation: a retrospective
comparison of desflurane and total intravenous anesthesia by target-controlled infusion under auditory evoked
potential guide.
Acta Anaesthesiol Taiwan. 2014;52(1):6-
12.
|
| 12 |
Qiu Q, Choi SW, Wong SSC, Irwin MG, Cheung CW.
Effects of intra-operative maintenance of general anaesthesia
with propofol on postoperative pain outcomes—
a systematic review and meta-analysis.
Anaesthesia.
2016;71(10):1222-1233.
|
| 13 |
Lin WL, Lee MS, Wong CS, et al.
Effects of intraoperative
propofol-based total intravenous anesthesia on postoperative
pain in spine surgery: comparison with desflurane
anesthesia—a randomised trial.
Medicine (Baltimore).
2019;98(13):e15074.
|
| 14 |
Zhang Y, Shan GJ, Zhang YX, et al; First Study of Perioperative
Organ Protection (SPOP1) investigators.
Propofol
compared with sevoflurane general anaesthesia is associated
with decreased delayed neurocognitive recovery
in older adults.
Br J Anaesth. 2018;121(3):595–604.
|
| 15 |
Wu ZF, Lee MS, Wong CS, et al.
Propofol-based total
intravenous anesthesia is associated with better survival
than desflurane anesthesia in colon cancer surgery.
Anesthesiology. 2018;129(5):932-941.
|
| 16 |
Lai HC, Lee MS, Lin C, et al.
Propofol-based total intravenous
anaesthesia is associated with better survival than
desflurane anaesthesia in hepatectomy for hepatocellular
carcinoma: a retrospective cohort study.
Br J Anaesth.
2019;123(2):151-160.
|
| 17 |
Lai HC, Lee MS, Lin KT, et al.
Propofol-based total intravenous
anesthesia is associated with better survival than
desflurane anesthesia in intrahepatic cholangiocarcinoma
surgery.
Medicine (Baltimore). 2019;98(51):e18472.
|
| 18 |
Lai HC, Lee MS, Liu YT, et al.
Propofol-based intravenous
anesthesia is associated with better survival than desflurane
anesthesia in pancreatic cancer surgery.
PLoS One.
2020;15(5):e0233598.
|
| 19 |
Lai HC, Lee MS, Lin KT, et al.
Propofol-based total intravenous
anesthesia is associated with better survival than
desflurane anesthesia in robot-assisted radical prostatectomy.
PLoS One. 2020;15(3):e0230290.
|
| 20 |
Huang NC, Lee MS, Lai HC, et al.
Propofol-based total intravenous
anesthesia improves survival compared to desflurane
anesthesia in gastric cancer surgery: a retrospective
analysis.
Medicine (Baltimore). 2020;99(25):e20714.
|
| 21 |
Huang YH, Wu ZF, Lee MS, et al.
Propofol-based total
intravenous anesthesia is associated with better survival
than desflurane anesthesia in glioblastoma surgery.
PLoS One. 2021;16(8):e0255627.
|
| 22 |
Huang YH, Lee MS, Lou YS, et al.
Propofol-based total
intravenous anesthesia did not improve survival compared
to desflurane anesthesia in breast cancer surgery.
PLoS One. 2019;14(11):e0224728.
|
| 23 |
Pandit JJ, Andrade J, Bogod DG, et al; Royal College
of Anaesthetists; Association of Anaesthetists of Great
Britain and Ireland.
5th National Audit Project (NAP5)
on accidental awareness during general anaesthesia:
summary of main findings and risk factors.
Br J Anaesth.
2014;113(4):549-559.
|
| 24 |
Wu KL, Wu ZF, Lai MF, et al.
A 10-year retrospective
analysis on the incidence of anesthesia awareness with recall
in adult patients under total intravenous anesthesia.
J
Med Sci. 2020;40(4):181-186.
|
| 25 |
Nimmo AF, Absalom AR, Bagshaw O, et al.
Guidelines for
the safe practice of total intravenous anaesthesia (TIVA):
joint guidelines from the Association of Anaesthetists
and the Society for Intravenous Anaesthesia.
Anaesthesia.
2019;74(2):211-224.
|
| 26 |
Absalom AR, Glen JIB, Zwart GJC, Schnider TW, Struys
MMRF.
Target-controlled infusion: a mature technology.
Anesth Analg. 2016;122(1):70-78.
|
| 27 |
Schnider TW, Minto CF, Struys MMRF, Absalom AR.
The safety of target-controlled infusions.
Anesth Analg.
2016;122(1):79-85.
|
| 28 |
Absalom AR, Mani V, De Smet T, Struys MMRF.
Pharmacokinetic
models for propofol—defining and illuminating
the devil in the detail.
Br J Anaesth. 2009;103(1):26-37.
|
| 29 |
Kataria BK, Ved SA, Nicodemus HF, et al.
The pharmacokinetics
of propofol in children using three different data
analysis approaches.
Anesthesiology. 1994;80(1):104-122.
|
| 30 | |
| 31 |
Eleveld DJ, Proost JH, Cortínez LI, Absalom AR, Struys
MMRF.
A general purpose pharmacokinetic model
for propofol.
Anesth Analg. 2014;118(6):1221-1237.
|
| 32 |
Nightingale CE, Margarson MP, Shearer E, et al.
Peri-operative
management of the obese surgical patient 2015:
Association of Anaesthetists of Great Britain and Ireland
Society for Obesity and Bariatric Anaesthesia.
Anaesthesia.
2015;70(7):859-876.
|
| 33 |
Scott HB, Choi SW, Wong GTC, Irwin MG.
The effect of
remifentanil on propofol requirements to achieve loss of
response to command vs. loss of response to pain.
Anaesthesia.
2017;72(4):479-487.
|
| 34 |
Yu EHY, Tran DHD, Lam SW, Irwin MG.
Remifentanil
tolerance and hyperalgesia: short-term gain, long-term
pain?
Anaesthesia. 2016;71(11):1347-1362.
|
| 35 |
Comelon M, Raeder J, Stubhaug A, Nielsen CS, Draegni
T, Lenz H.
Gradual withdrawal of remifentanil infusion
may prevent opioid-induced hyperalgesia.
Br J Anaesth.
2016;116(4):524-530.
|
| 36 |
Wu TS, Wu HC, Wu ZF, Huang YH.
Nalbuphine sebacate
interferes with the analgesic effect of fentanyl.
Med Sci.
2020;40(2):101-102.
|
| 37 |
Zecha-Stallinger A, Schmölz W, Wenzel V.
The three-way
stopcock may be a weak component of total intravenous
anaesthesia.
Acta Anaesthesiol Scand. 2009;53(9):1173-
1175.
|
| 38 |
Wigmore TJ, Mohammed K, Jhanji S.
Long-term survival
for patients undergoing volatile versus IV anesthesia for
cancer surgery: a retrospective analysis.
Anesthesiology.
2016;124(1):69-79.
|
| 39 | |
| 40 |
Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol
N.
Bispectral index for improving anaesthetic delivery
and postoperative recovery.
Cochrane Database Syst
Rev. 2014:CD003843.
|
| 41 |
Vasile B, Rasulo F, Candiani A, Latronico N.
The pathophysiology
of propofol infusion syndrome: a simple
name for a complex syndrome.
Intensive Care Med.
2003;29(9):1417-1425.
|
| 42 |
Hajat Z, Ahmad N, Andrzejowski J.
The role and limitations
of EEG-based depth of anaesthesia monitoring in
theatres and intensive care.
Anaesthesia. 2017;72(Suppl
1):38-47.
|
| 43 |
Barker N, Lim J, Amari E, Malherbe S, Ansermino JM.
Relationship between age and spontaneous ventilation during intravenous anesthesia in children.
Paediatr
Anaesth. 2007;17(10):948-955.
|
| 44 |
Muñoz HR, Cortínez LI, Ibacache ME, Altermatt FR.
Remifentanil requirements during propofol administration
to block the somatic response to skin incision in
children and adults
Anesth Analg. 2007;104(1):77-80.
|
| 45 |
Constant I, Sabourdin N.
The EEG signal: a window on the
cortical brain activity.
Paediatr Anaesth. 2012;22(6):539-
552.
|
| 46 |
Kang JG, Kim JK, Jeong HS, et al.
A prospective, randomized
comparison of the effects of inhaled sevoflurane
anesthesia and propofol/remifentanil intravenous anesthesia
on salivary excretion during laryngeal microsurgery.
Anesth Analg. 2008;106(6):1723-1727.
|
| 47 |
Lai HC, Tai HW, Wu ZF.
Excess nasopharyngeal secretions
in ocular surgery under propofol-based total
intravenous anesthesia.
J Clin Anesth. 2016;34:257-258.
|
| 48 |
Lai HC, Tseng WC, Pao SI, et al.
Relationship between
anesthesia and postoperative endophthalmitis: a retrospective
study.
Medicine (Baltimore). 2017;96(12):e6455.
|
| 49 |
Lai HC, Chang YH, Huang RC, et al.
Efficacy of sevoflurane
as an adjuvant to propofol-based total intravenous
anesthesia for attenuating secretions in ocular surgery.
Medicine (Baltimore). 2017;96(17):e6729.
|
| 50 |
Lai HC, Huang TW, Tseng WC, Lin WL, Chang H, Wu
ZF.
Sevoflurane is an effective adjuvant to propofol-based
total intravenous anesthesia for attenuating cough reflex
in nonintubated video-assisted thoracoscopic surgery./b>
Medicine (Baltimore). 2018;97(42):e12927.
|