Abstract
Anesthesia for patients with morbid obesity can be challenging because of increased risk of opioid-related adverse events, postoperative nausea and vomiting (PONV), and poor pain control. We conducted a systematic review and meta-analysis to compare the safety and efficacy of total intravenous anesthesia (TIVA) with inhalation anesthesia in patients undergoing bariatric surgery. We searched MEDLINE, EMBASE, CENTRAL, and the Clinical Trials Registry database from inception to July 22, 2020. Primary outcomes were postoperative pain and PONV scores. Secondary outcomes included opioid requirements, intraoperative time, complications, and time to recovery. Grading of Recommendations Assessment, Development, and Evaluation framework was used to rate the certainty of evidence. Among 722 studies identifi ed in our search, 7 randomized studies involving a total of 682 patients met the inclusion criteria. Bariatric surgery with TIVA resulted in a lower incidence of nausea (relative risk [RR], 0.54; 95% CI, 0.31–0.94; p = 0.03; moderate certainty) and vomiting (RR, 0.31; 95% CI, 0.13–0.74; p = 0.008; moderate certainty). There was no difference in postoperative pain at 30 minutes, 1 hour, or 24 hours, or in postoperative opioid requirements. Patients undergoing bariatric surgery with TIVA had significantly lower incidence of PONV but no difference in postoperative pain when TIVA was compared to inhalation anesthesia techniques. These benefi ts should be considered in order to improve the quality of care and enhance recovery for the bariatric population, who are at an increased baseline risk of perioperative complications. Future adequately powered randomized controlled trials are needed to compare the effi cacy of the anesthesia regimens in patients undergoing bariatric surgery.
Keywords
bariatric surgery, PONV, TIVA
Introduction
Obesity is a public health concern with more than 650 million adults worldwide meeting the criteria for being obese (body mass index > 30 kg/m2).1 Over one-third of American adults were considered obese in 2015–2016, and the number of obese adults continues to rise at alarming levels.2-4 Obesity is associated with increased morbidity and mortality, as well as the development of chronic health conditions like cardiovascular disease, metabolic syndrome, cancer, osteoarthritis, or obstructive sleep apnea.5
Bariatric surgery remains the most effective treatment in morbidly obese individuals, resulting in sustained long-term weight loss and resolution of obesity- associated comorbidities such as type 2 diabetes, non-alcoholic fatty liver disease, cardiovascular disease, and systemic inflammation.6 However, bariatric surgery poses several challenges to anesthesiologists in the perioperative period. Specific considerations in the immediate postoperative period include increased risk of postoperative nausea and vomiting (PONV), poor pain control, and prolonged recovery.7,8
Total intravenous anesthesia (TIVA) and inhalation anesthesia, a combination of intravenous and inhalation agents, are commonly used in bariatric surgery. Each regimen has its inherent advantages and disadvantages. While volatile anesthetics are known to cause PONV, TIVA is associated with possible hyperalgesia and is more expensive.9 However, TIVA has displayed faster cognitive recovery, improved time to discharge, and easier emergence from anesthetic use.10 Currently, it is unclear which anesthetic regimen is optimal for patients undergoing bariatric surgery for enhanced recovery in the postoperative period.
Given the higher risk of poorer outcomes in these population and unclear distinction between these two popular anesthetic regimens, we conducted a comprehensive systematic review and meta-analysis of all studies comparing the use of the inhalation anesthesia technique with the TIVA technique in patients undergoing bariatric surgery to determine their safety and efficacy in the postoperative period.
Materials and Methods
Information Sources
Eligible studies were found through a systematic search of MEDLINE, EMBASE, and CENTRAL, from the inception of each database to July 22, 2020. The keywords that we searched include “bariatric surgery,” “total intravenous anesthesia,” and similar phrases (complete search strategies are available in Supplement Tables 1 and 2). References from both published studies and gray literature were included in our search to ensure that relevant articles were not omitted, and we also screened the bibliographies of included studies for relevant studies. We did not discriminate against full texts by language. The systematic review and meta-analysis were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1).11
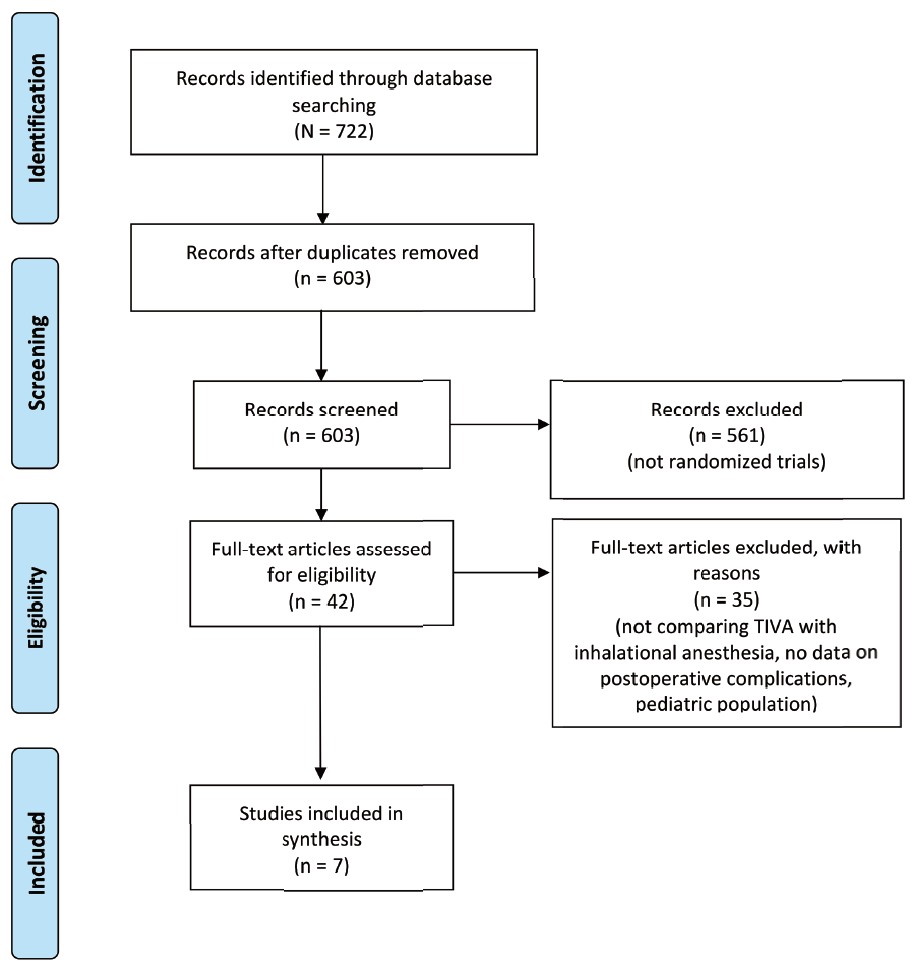
Download full-size image
Abbreviation: TIVA, total intravenous anesthesia.
aFor more information, visit: http://www.prisma-statement.org/
Eligibility Criteria and Data Abstraction
Articles were eligible for inclusion if the studies compared the use of TIVA with inhalation anesthesia in patients undergoing bariatric surgery. We restricted inclusion to only randomised controlled trials (RCTs) as identified by the authors of each study. Exclusion criteria included (1) letters, editorials, review articles; (2) nonhuman studies; (3) pediatric populations; and (4) studies without mention of intravenous or inhalation anesthesia.
Two reviewers (M.M.A. and J.L.) independently screened the searched titles, abstracts, and full texts in accordance with pre-defined inclusion and exclusion criteria. Reviewers were unblinded to authors, institution, or journal of publication. Any discrepancies that occurred at the title and abstract screening stages were resolved by automatic inclusion to ensure all relevant papers were not missed. Discrepancies at the full-text screening stage were resolved by consensus between the two reviewers and if the disagreement persisted, a third reviewer was consulted for resolution (C.T). Two reviewers (M.M.A. and J.L.) independently performed data abstraction onto a standardized spreadsheet designed a priori. The following variables were abstracted from included studies: (1) study characteristics (authors, country, year of publication, and study design); (2) patient demographic characteristics (mean age at the time of surgery, sex, and number of patients), co-morbidities; (3) primary outcomes: pain score, PONV score; and (4) secondary outcomes: intraoperative opioid use, follow-up time periods, and surgical outcomes. Any disagreements between reviewers were resolved by consensus or by consulting a third reviewer.
Risk of Bias Assessment
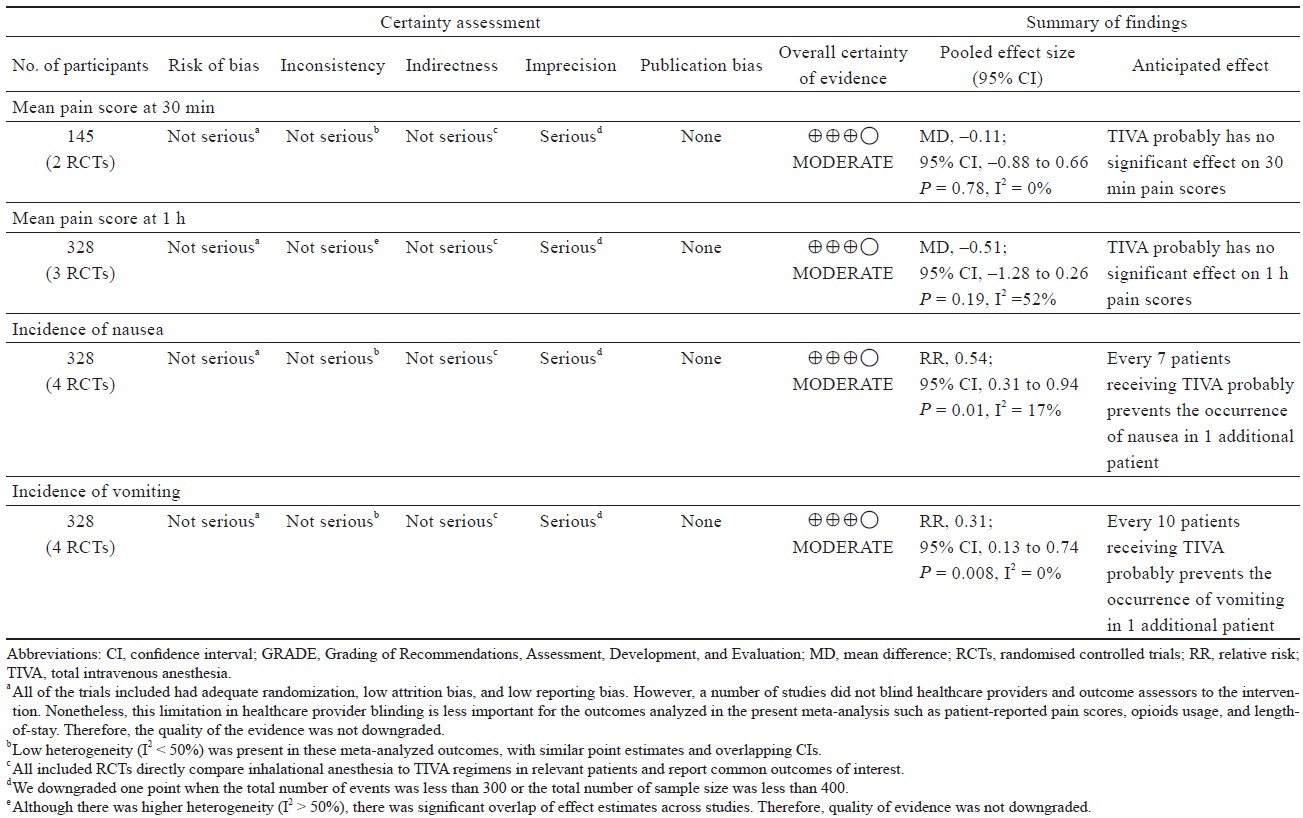
The quality of eligible RCTs was assessed using the Cochrane risk of bias tool for randomized trials.12 Two authors independently assessed the following risk of bias issues: (1) random sequence generation; (2) allocation concealment; (3) blinding of study participants, personnel, and outcome assessors; (4) incomplete outcome data (≥ 20% missing data was considered at high risk of bias); and (5) performance bias. Any discrepancy in the assessment of risk of bias was resolved by discussion or third-party adjudication if needed. Funnel plots were generated to assess potential publication bias for meta-analysis containing at least 10 studies as fewer studies can lead to bias when distinguishing symmetry and asymmetry in the funnel plot.13 Certainty of evidence for estimates derived from each meta-analyzed outcome from RCTs was assessed by the Grading of Recommendations, Assessment, Development, and Evaluation.14
Statistical Analysis
Pain scores of different scales (numeric rating scale or visual analogue scale) were converted into a common 0–10 scale (0 = no pain, 10 = worst pain experienced by the patient). Incidence of PONV in the post-anesthesia care unit was collected as a dichotomous outcome. We performed pairwise meta-analyses by using a DerSimonian and Laird random-effects model for continuous and dichotomous variables. For dichotomous outcomes, we combined the relative risk (RR) and 95% confidence intervals (CIs) by using inverse variance method with random-effects models. Statistical heterogeneity was assessed by using chisquared tests and I-squared statistics. All primary analyses were performed with STATA v15.1 (Stata Corp, College Station, TX, USA).
Results
Of 603 unique search results, 42 articles were identified for full-text evaluation, and 7 randomized studies were included for meta-analysis.15-21 Reasons for exclusion are documented in Figure 1.11
Study Characteristics
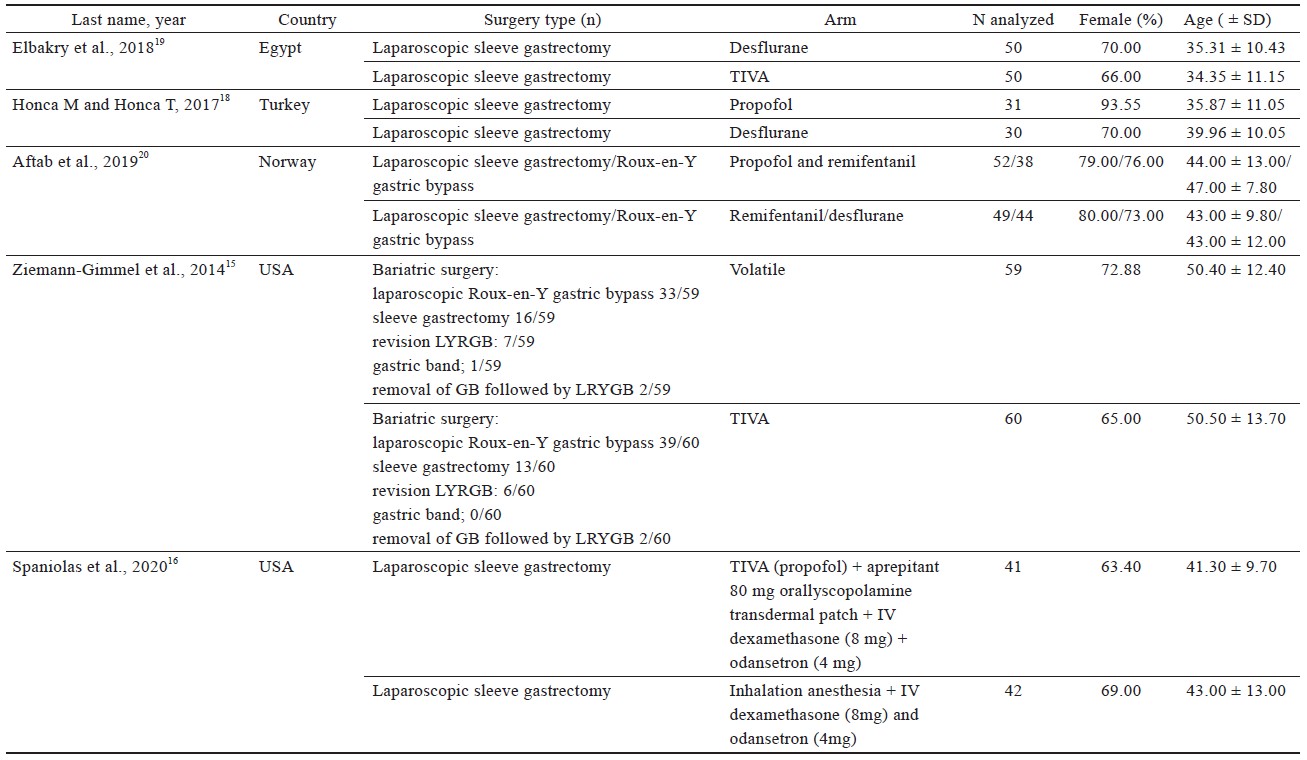
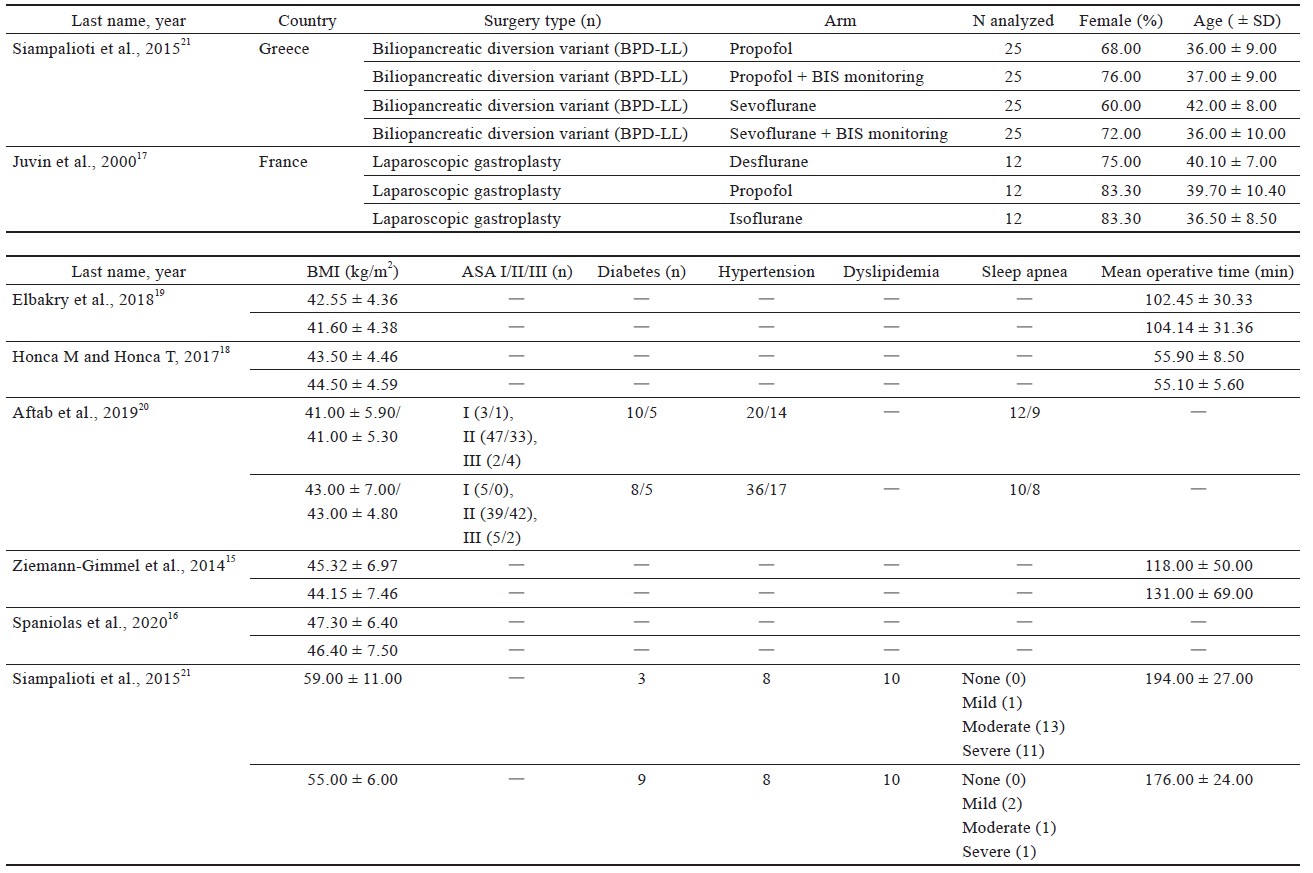
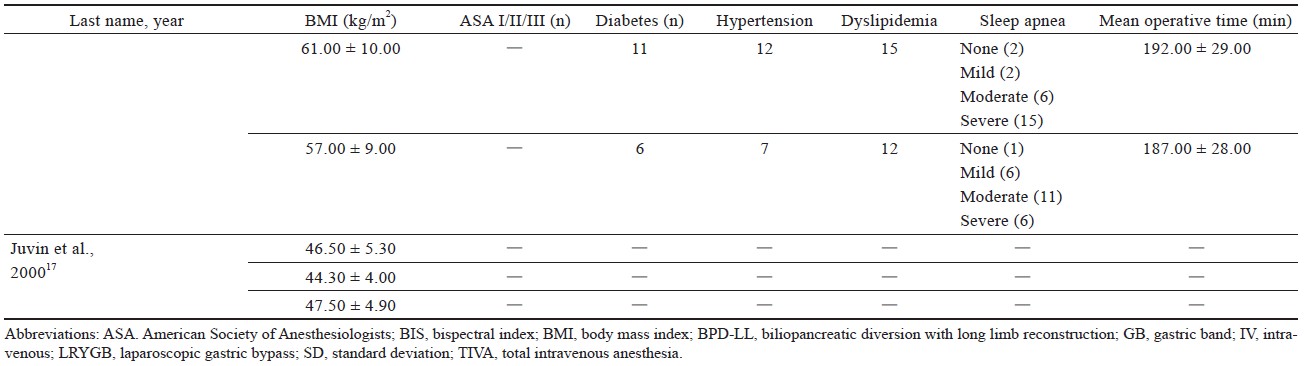
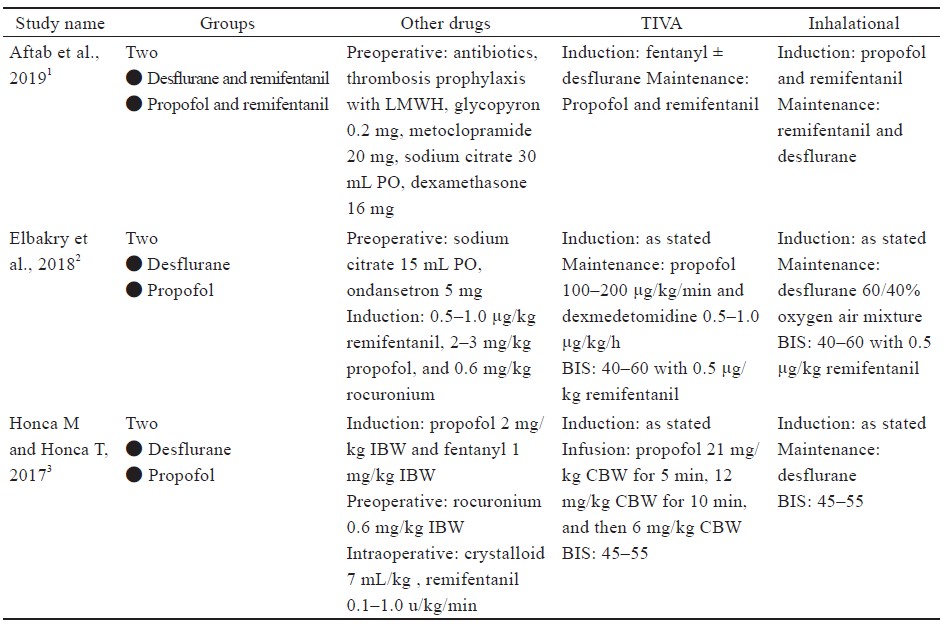
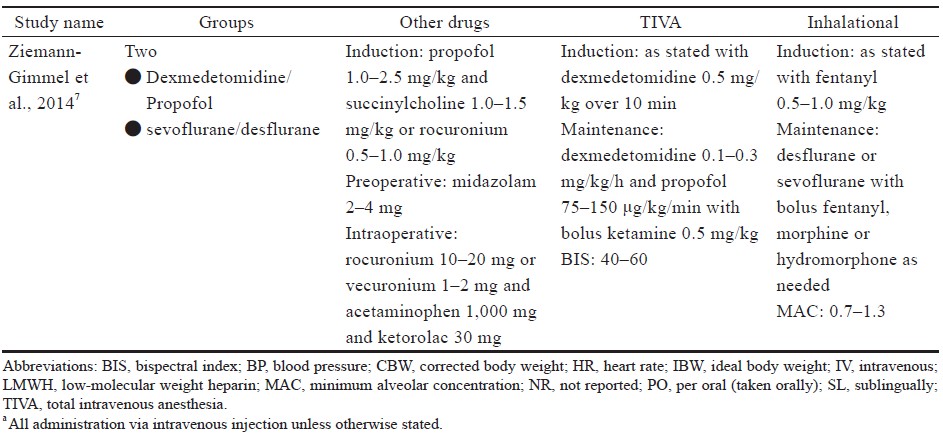
Study characteristics are outlined in Table 1.15-21 Comorbidities that were reported include hypertension, diabetes, sleep apnea, dyslipidemia of American Society of Anesthesiologists’ classification, and sleep apnea (Table 1).15-21
Download full-size image
Download full-size image
Download full-size image
Four studies16,18-20 used laparoscopic sleeve gastrectomy (LSG) (n = 345); one study15 used sleeve gastrectomy (n = 29); two studies15,20 used laparoscopic Roux-en-Y gastric bypass (LRYGB) (n = 154) and gastric bypass (n = 72); one study21 used biliopancreatic diversion (n = 100), gastric band surgery (n = 1), and LRYGB with gallbladder removal (n = 4). The trial by Ziemann-Gimmel et al.15 compared anesthetic techniques from multiple forms of bariatric surgery and reported an aggregate outcome, whereas the study by Aftab et al.20 reported separate results for each form of bariatric surgery.
Anesthesia Regimens
Specific descriptions of anesthetic regimens, including induction, maintenance, minimum alveolar concentration, bispectral index (BIS) monitoring, and other medications for each included study are in Supplement Table 3. Medications were used for both groups unless specific descriptions were listed under the respective study’s TIVA or inhalation section.
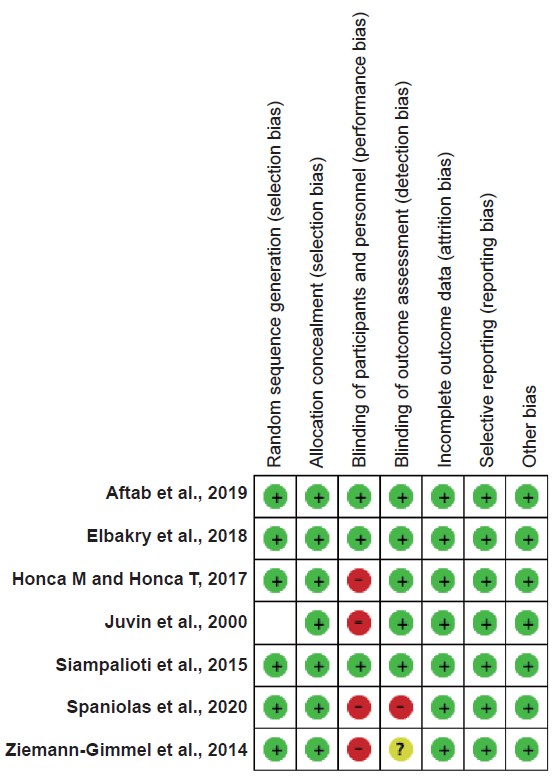
Risk of Bias
The risk of bias results is displayed in Supplement Figure 1. The most common sources of potential bias were blinding healthcare providers and outcome assessment. There was no explicit report of how groups were blinded in four studies.15-18
Postoperative Pain and Postoperative Opioid Use
Postoperative pain was reported at 30 minutes, 1 hour, and 24 hours postoperatively while postoperative opioid use was reported as 24-hour total opioid requirement in oral morphine equivalents. Pooling of outcomes revealed no significantly different effect in pain scores of TIVA versus control groups at 30 minutes (Figure 2A; Table 2), 1 hour (Figure 2B; Table 2), 24 hours (Figure 2C) and postoperative opioid use (Figure 3).15,17,19-21
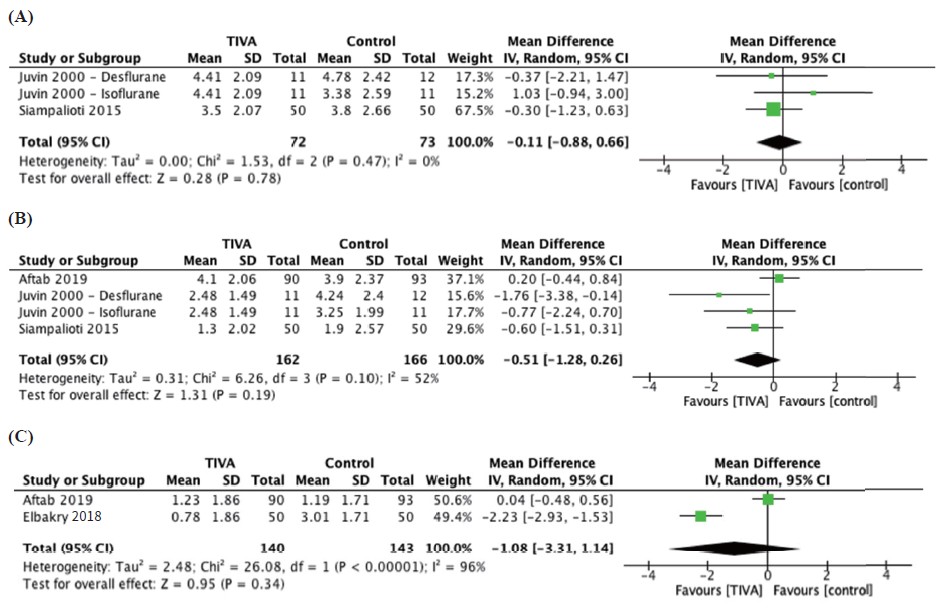
Download full-size image
Abbreviations: CI, confi dence interval; IV, intravenous; SD, standard deviation.
Download full-size image
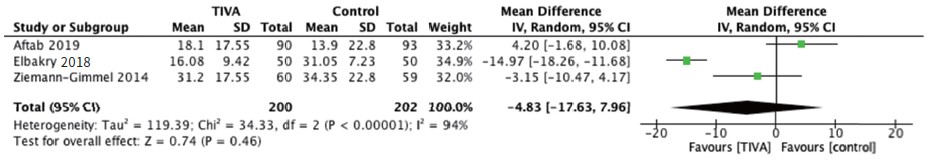
Download full-size image
Abbreviations: CI, confi dence interval; IV, intravenous; SD, standard deviation.
Decrease in PONV
Nausea was reported by four studies with a total of 165 participants in the TIVA group and 163 participants in the control group. The pooled effect size showed a significantly decreased incidence of nausea in the TIVA group, corresponding to a number needed to treat (NNT) of 7 (RR = 0.54, 95% CI 0.31, 0.94, I2 = 17%; P = 0.03; Figure 4A15,17-19; Table 2; moderate quality).
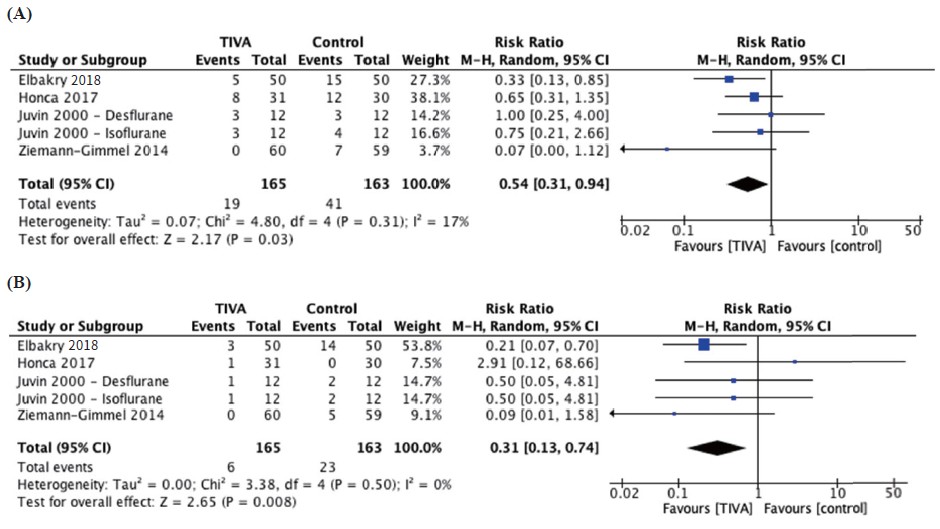
Download full-size image
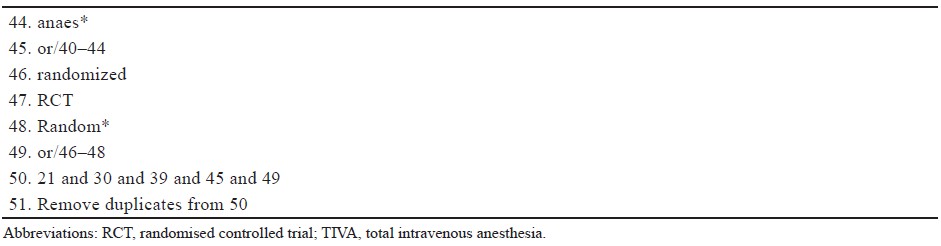
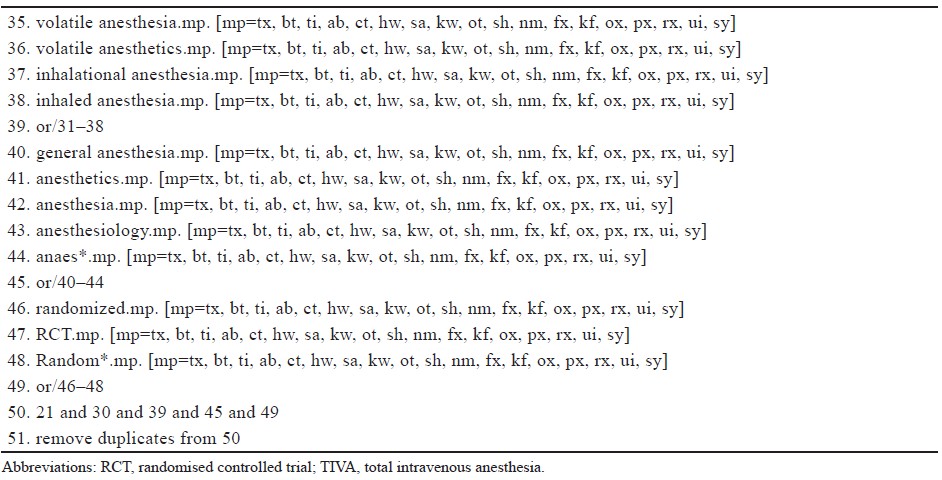
Abbreviations: CI, confi dence interval.
Vomiting was reported by five studies with a total of 165 TIVA subjects and 163 control subjects and was significantly reduced in the TIVA group compared to the inhalational group, with a NNT of 10 (RR = 0.31, 95% CI 0.13, 0.74, I2 = 0%; P = 0.008; Figure 4B15,17-19; Table 2; moderate certainty).
Discussion
To our knowledge, the present study is the first comprehensive systematic review and meta-analysis of the existing literature of the choice of anesthetic regimens, TIVA versus inhalation anesthesia based on postoperative pain and PONV after bariatric surgery. PONV can precipitate serious adverse events, and patients often rate PONV as worse than postoperative pain.22 In addition to prolonged discharge, vomiting or retching can result in life-threatening outcomes such as wound dehiscence, esophageal rupture, aspiration, dehydration, increased intracranial pressure, and pneumothorax.23
Analysis of the data suggests that TIVA reduced PONV (NNT = 7; moderate certainty), compared to inhalation anesthesia, with no difference in pain scores or opioid consumption. The RR reduction of 46% for postoperative nausea and 69% for postoperative vomiting are much higher than the 39% found by Schraag et al.24 This difference in effect size is also noted when being compared to Kumar et al.25, who similarly found a 44% and 50% reduction in PONV, respectively. However, it should be noted that the aforementioned authors did not exclusively look at bariatric surgery patients which may suggest that the reduction of PONV in TIVA varies according to different surgical settings and requires further investigation.24,25
Treating PONV incurs significant costs. Also, though it remains controversial, morbidly obese patients undergoing bariatric surgery are at higher risk of suffering from PONV compared to non-morbidly obese patients.26 An economic analysis demonstrated that PONV prophylaxis was favorable when being weighed against the expenses of returning to the hospital with PONV. If prophylaxis were administered to all surgical patients during the study timeframe, it might save to the tune of over $100K.27 Therefore, employing an anesthetic regimen that might be protective against PONV may also be economically efficient, particularly of concern in patients undergoing bariatric surgery who may be resistance to PONV prophylaxis and present higher incidences of PONV than the general population.28 These results prompt investigation and evaluation of adjuncts to prevent PONV for patients undergoing bariatric surgery.29
The findings of the present review agree with previous high-powered reviews comparing the use of TIVA with inhalational anesthesia. Other reviews also demonstrate a significant decrease in the incidence of PONV with TIVA for ambulatory surgery.24,25 We found no difference between anesthetic techniques for other outcomes such as postoperative surgical or anesthetic complications, pain scores at various time points, or hospital length of stay.
The findings in our study should be interpreted considering the following limitations. The main limitation of our review is the limited number of studies available for analysis because we restricted our inclusion to only RCTs as they are the gold standard for analysis when there is uncertainty in the superiority of one intervention versus another.30 Furthermore, the reported outcomes of the studies included were inconsistent, for only two studies reported their use of antiemetic medications,15,20 and only one of them had statistically significant results with patients undergoing TIVA receiving significantly fewer antiemetics than those undergoing inhalational anesthesia.20 Also,induction was inconsistent between some groups, with one study not reporting any specifi cs (Supplement Table 3).16 There was also substantial heterogeneity in the results for pain at 1 hour and at 24 hours, suggesting signifi cant variances between studies that influence the reliability of meta-analyzed outcomes. Causes of the heterogeneity in these studies may include operative differences (5 laparoscopic ports for LSG and 6 for LRYGB, operative time), variation in TIVA (drugs used, infusions rate) and inhalation anesthetic (sevoflurane, desflurane), and differences in intraoperative monitoring and targeting to allow for effective titration of each anesthetic technique (such as BIS, minimal alveolar concentration). The granular detail was not available in these studies, and therefore unable to be stratifi ed in subgroup analysis to account for study heterogeneity. Lastly, there was a lack of recovery related outcomes available for meta-analysis (such as length of hospital stay, time to ambulation, first flatus, and first solid oral intake).
In conclusion, our systematic review and meta- analysis of RCTs suggest that compared to inhalation anesthesia, TIVA reduces the occurrence of PONV in patients undergoing bariatric surgery. In a population particularly susceptible to PONV, our evidence suggests the superiority of TIVA over volatile anesthetics with moderate certainty. Future rigorous and larger randomized trials are needed to investigate the variety of measures to assess recovery after surgery in this high-risk surgical population.
Conflict of Interest
No potential confl ict of interest relevant to this article was reported.
Funding
None.
References
| 1 | |
| 2 |
Hurt RT, Kulisek C, Buchanan LA, McClave SA.
The obesity obesity
epidemic: challenges, health initiatives, and implications
for gastroenterologists.
Gastroenterol Hepatol (N Y).
2010;6(12):780-792.
|
| 3 |
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden
CL.
Trends in obesity and severe obesity prevalence
in US youth and adults by sex and age, 2007–2008 to
2015–2016.
JAMA. 2018;319(16):1723-1725.
|
| 4 | |
| 5 | |
| 6 |
Wolfe BM, Kvach E, Eckel RH.
Treatment of obesity: weight
loss and bariatric surgery.
Circ Res. 2016;118(11):1844-
1855.
|
| 7 |
Ferreira AT, Duarte NMC, Caetano AMM, et al.
Postoperative
pain following bariatric surgery: correlation
between intensity and clinical-surgical variables.
Bariatr
Surg Pract Patient Care. 2019;14(2):57-61.
|
| 8 |
Adis Medical Writers.
Manage perioperative pain in
morbidly obese patients by taking an all-round multimodal
approach.
Drugs Ther Perspect. 2020;36:139-145.
|
| 9 |
Smith I.
Total intravenous anaesthesia: is it worth the cost?.
CNS Drugs. 2003;17(9):609-619.
|
| 10 |
Kocaturk O, Keles S.
Recovery characteristics of total
intravenous anesthesia with propofol versus sevoflurane
anesthesia: a prospective randomized clinical trial.
J Pain
Res. 2018;11:1289-1295.
|
| 11 |
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA
Group.
Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement.
PLoS Med.
2009;6(7):e1000097.
|
| 12 |
Sterne JAC, Savović J, Page MJ, et al.
RoB 2: a revised
tool for assessing risk of bias in randomised trials.
BMJ.
2019;366:l4898.
|
| 13 |
Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin
I.
The case of the misleading funnel plot.
BMJ.
2006;333(7568):597-600.
|
| 14 |
Page MJ, Higgins JPT, Sterne JAC.
Chapter 13: Assessing
risk of bias due to missing results in a synthesis.
In: Higgins
JPT, Thomas J, Chandler J, Cumpston M, Li T, Page
MJ Welch V, eds. Cochrane Handbook for Systematic
Reviews of Interventions version 6.1. London, England:
Cochrane; 2020.
|
| 15 |
Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema
RT.
Opioid-free total intravenous anaesthesia reduces
postoperative nausea and vomiting in bariatric surgery
beyond triple prophylaxis.
Br J Anaesth. 2014;112(5):906-
911.
|
| 16 |
Spaniolas K, Nie L, Moller D, et al.
A comprehensive
approach for the prevention of nausea and vomiting following
sleeve gastrectomy: a randomized controlled trial.
Obes Surg. 2020;30(11):4250-4257.
|
| 17 |
Juvin P, Vadam C, Malek L, Dupont H, Marmuse JP,
Desmonts JM.
Postoperative recovery after desflurane,
propofol, or isoflurane anesthesia among morbidly obese
patients: a prospective, randomized study.
Anesth Analg.
2000;91(3):714-719.
|
| 18 |
Honca M, Honca T.
Comparison of propofol with desflurane
for laparoscopic sleeve gastrectomy in morbidly
obese patients: a prospective randomized trial.
Bariatr
Surg Pract Patient Care. 2017;12(2):49-54.
|
| 19 |
Elbakry AE, Sultan WE, Ibrahim E.
A comparison between
inhalational (Desflurane) and total intravenous
anaesthesia (Propofol and dexmedetomidine) in improving
postoperative recovery for morbidly obese patients
undergoing laparoscopic sleeve gastrectomy: a double-
blinded randomised control.
J Clin Anesth. 2018;45:6-
11. doi
|
| 20 |
Aftab H, Fagerland MW, Gondal G, Ghanima W, Olsen
MK, Nordby T.
Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia:
a double blind, randomized, controlled trial.
Surg Obes Relat Dis. 2019;15(9):1505-1512.
|
| 21 |
Siampalioti A, Karavias D, Zotou A, Kalfarentzos F, Filos
K.
Anesthesia management for the super obese: is sevoflurane
superior to propofol as a sole anesthetic agent? A
double-blind randomized controlled trial.
Eur Rev Med
Pharmacol Sci. 2015;19(13):2493-2500.
|
| 22 |
Macario A, Weinger M, Carney S, Kim A.
Which clinical
anesthesia outcomes are important to avoid? The perspective
of patients.
Anesth Analg. 1999;89(3):652-658.
|
| 23 |
Maitra S, Som A, Baidya DK, Bhattacharjee S.
Comparison
of ondansetron and dexamethasone for prophylaxis
of postoperative nausea and vomiting in patients
undergoing laparoscopic surgeries: a meta-analysis of
randomized controlled trials.
Anesthesiol Res Pract.
2016;2016:7089454.
|
| 24 |
Schraag S, Pradelli L, Alsaleh AJO, et al.
Propofol vs.
inhalational agents to maintain general anaesthesia in
ambulatory and in-patient surgery: a systematic review
and meta-analysis.
BMC Anesthesiol. 2018;18(1):162.
|
| 25 |
Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D.
A
comparison of total intravenous anaesthesia using propofol
with sevoflurane or desflurane in ambulatory surgery:
systematic review and meta-analysis.
Anaesthesia.
2014;69(10):1138-1150.
|
| 26 |
Groene P, Eisenlohr J, Zeuzem C, Dudok S, Karcz K,
Hofmann-Kiefer K.
Postoperative nausea and vomiting
in bariatric surgery in comparison to non-bariatric
gastric surgery.
Wideochir Inne Tech Maloinwazyjne.
2019;14(1):90-95.
|
| 27 |
Dzwonczyk R, Weaver TE, Puente EG, Bergese SD.
Postoperative nausea and vomiting prophylaxis from an
economic point of view.
Am J Ther. 2012;19(1):11-15.
|
| 28 |
Nazar CE, Lacassie HJ, López RA, Muñoz HR.
Dexamethasone
for postoperative nausea and vomiting prophylaxis:
effect on glycaemia in obese patients with impaired glucose
tolerance.
Eur J Anaesthesiol. 2009;26(4):318-321.
|
| 29 |
Halliday TA, Sundqvist J, Hultin M, Walldén J.
Post-operative
nausea and vomiting in bariatric surgery patients:
an observational study.
Acta Anaesthesiol Scand.
2017;61(5):471-479.
|
| 30 |
Djulbegovic B.
Uncertainty and equipoise: at interplay
between epistemology, decision making and ethics.
Am J Med Sci. 2011;342(4):282-289.
|
Supplement

Download full-size image
Download full-size image

Download full-size image
Download full-size image
Download full-size image

Download full-size image
Download full-size image
Download full-size image
Abbreviation: TIVA, total intravenous anesthesia.
Supplementary References
| 1 |
Aftab H, Fagerland MW, Gondal G, Ghanima W, Olsen MK, Nordby T.
Pain and nausea after bariatric surgery
with total intravenous anesthesia versus desflurane anesthesia: a double blind, randomized, controlled trial.
Surg
Obes Relat Dis. 2019;15(9):1505-1512.
|
| 2 |
Elbakry AE, Sultan WE, Ibrahim E.
A comparison between inhalational (Desflurane) and total intravenous anaesthesia
(Propofol and dexmedetomidine) in improving postoperative recovery for morbidly obese patients
undergoing laparoscopic sleeve gastrectomy: a double-blinded randomised control.
J Clin Anesth. 2018;45:6-11.
|
| 3 |
Honca M, Honca T.
Comparison of propofol with desflurane for laparoscopic sleeve gastrectomy in morbidly
obese patients: a prospective randomized trial.
Bariatr Surg Pract Patient Care. 2017;12(2):49-54.
|
| 4 |
Juvin P, Vadam C, Malek L, Dupont H, Marmuse JP, Desmonts JM.
Postoperative recovery after desflurane,
propofol, or isoflurane anesthesia among morbidly obese patients: a prospective, randomized study.
Anesth Analg.
2000;91(3):714-719.
|
| 5 |
Siampalioti A, Karavias D, Zotou A, Kalfarentzos F, Filos K.
Anesthesia management for the super obese: is sevoflurane
superior to propofol as a sole anesthetic agent? A double-blind randomized controlled trial.
Eur Rev Med
Pharmacol Sci. 2015;19(13):2493-2500.
|
| 6 |
Spaniolas K, Nie L, Moller D, et al.
A comprehensive approach for the prevention of nausea and vomiting following
sleeve gastrectomy: a randomized controlled trial.
Obes Surg. 2020;30(11):4250-4257.
|
| 7 |
Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT.
Opioid-free total intravenous anaesthesia reduces
postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis.
Br J Anaesth. 2014;112(5):906-
911.
|