Abstract
Background: Preemptive analgesia is important for reducing postoperative analgesia requirement. Therefore, this study compared the efficacy of intravenous (IV) ketamine alone with the efficacy of a combination of low-dose IV ketamine and IV parecoxib as part of a multimodal preemptive analgesia regimen in patients undergoing elective laparotomy.
Methods: In this prospective study, 48 patients scheduled for elective laparotomy were randomized to two groups of preemptive analgesia, namely, group K-P, in which anestheologists administered a combination of 0.3 mg/kg IV ketamine and 40.0 mg IV parecoxib, or group K, in which ones gave 0.3 mg/kg IV ketamine alone. Patients from both groups underwent surgery under general anesthesia, and total intraoperative opioid requirement was recorded. After surgery, morphine administered by automated patient-controlled analgesia (PCA) infusion device was initiated in all patients. Pain score was assessed using the visual analogue scale (VAS), and postoperative opioid requirement was recorded at 1 and 4 hours, and subsequently from 4-hour intervals up to 24 hours after surgery.
Results: Compared to group K, group K-P required signifi cantly lower rescue IV fentanyl in the recovery bay (0.10 ± 0.28 vs. 0.35 ± 0.46 μg/kg; P = 0.031), showing prolonged time-to-fi rst analgesic request recorded by PCA device (70.8 ± 40.0 vs. 22.2 ± 15.8 mins; P < 0.001), lower total morphine requirement delivered by PCA device (8.0 ± 4.6 vs. 16.8 ± 6.5 mg; P < 0.001), and lower VAS values measured at all time points. There was no signifi cant difference in intraoperative total opioid requirement between the groups.
Conclusions: Among laparotomy patients, multimodal preemptive analgesia by the use of a combination of low-dose IV ketamine and IV parecoxib was more effective than IV ketamine alone in reducing pain scores and postoperative analgesia requirement (e.g., PCA-administered morphine).
Keywords
analgesia, ketamine, morphine, multimodal, patient-controlled
Introduction
Preemptive analgesia refers to the administration of an analgesic before a surgical incision or tissue injury aimed at reducing or preventing post-procedure pain. Preemptive analgesia can reduce postoperative analgesia requirement and involve either intravenous (IV) pharmacological administration or provision of a relevant regional block. However, some of the drugs should be used carefully because of side effects. Multimodal analgesia is another concept in pain management wherein two or more drugs with different mechanisms of action are administered together to provide analgesia. These drugs may either be administered via the same route or different routes, and the aim is to provide pain relief with lowering opioid requirement and reducing opioid-related adverse effects. These two concepts can be combined during management of postoperative pain, as multimodal preemptive analgesia or as a combination of preemptive followed by intraoperative or postoperative multimodal analgesia.1,2 The aim of preemptive analgesia is to reduce central sensitisation from noxious inputs during the entire perioperative period.3
In literature, there is some confusion regarding the terms “preemptive multimodal analgesia” and “multimodal preemptive analgesia”. Preemptive multimodal analgesia refers to a combination of preemptive analgesia and multimodal analgesia during and after surgery,4 whereas multimodal preemptive analgesia refers to a combination of two or more modalities of only preemptive analgesia.5 However, many studies have also used the term preemptive multimodal analgesia to denote a combination of two or more modalities of preemptive analgesia.6,7 Multimodal and preemptive analgesia are also part of the “Enhanced Recovery After Surgery” (ERAS) protocol, which aims to facilitate early mobility and return of bowel function, apart from reducing postoperative morbidity.8
Ketamine is an N-methyl-D-aspartate receptor antagonist that is commonly used either as an IV induction agent, sedative agent, or as part of multimodal analgesia, and its use as preemptive analgesia has been described in a few studies. A meta-analysis based on data from 5 randomised controlled trials (RCTs) involving the use of ketamine for preemptive analgesia concluded that ketamine was effective in reducing postoperative morphine consumption and prolonging time to first analgesia request. Importantly, it was reported to be as safe as physiological saline with respect to side effects such as nausea and vomiting.9 However, previous studies have investigated only a few combinations of ketamine and other drugs as preemptive analgesia. For example, a combination of oral acetaminophen and gabapentin followed by IV ketamine and dexamethasone as preemptive analgesia can reduce pain safely and effectively in the early postoperative period following anorectal surgery.10
Parecoxib is the only IV cyclooxygenase-2 inhibitor that can be a beneficial analgesic for moderate- to-severe acute postoperative pain, and its use in preemptive analgesia has been described in a few studies. A combination of parecoxib and ultrasound- guided paravertebral block as preemptive analgesia during video-assisted thoracoscopic surgery brings about better pain relief, lower sufentanil and ketorolac consumption, greater haemodynamic stability, and lower surgery-related stress response.11The effectiveness of analgesia delivered by patient-controlled analgesia (PCA) device was to decrease visual analogue scale (VAS) values in the short term and to relieve pain shortly after total knee replacement surgery when anesthesiologists used it as a postoperative analgesia modality.12
To the best of our knowledge, no previous study compared IV ketamine alone with multimodal preemptive analgesia that combines IV ketamine and IV parecoxib in laparotomy patients. Only one study has compared preemptive IV ketamine with IV parecoxib for laparoscopic uterus surgery, and it showed that a single injection of short-acting ketamine before laparoscopic uterus surgery was as effective as long-acting parecoxib with respect to an opioid-sparing effect during the first 24 hours after surgery. However, parecoxib is known to have a better analgesic effect during the early postoperative period.13 Therefore, the aim of this study was to compare the efficacy of preemptive analgesia with IV ketamine alone and multimodal preemptive analgesia that combines low-dose IV ketamine and IV parecoxib. The primary outcome measures were intraoperative opioid requirement, postoperative pain scores evaluated by using the VAS, and postoperative opioid requirement in patients undergoing elective laparotomy.
Methods
Our study was a prospective, double-blinded, RCT. After receiving the approval from the Institutional Human Research Ethics Committee (approval code: USM/JEPeM/17080376) and the written informed consent from patients, we recruited 48 patients scheduled for elective laparotomy under general anesthesia who met the inclusion and exclusion criteria during pre-operative assessment. The inclusion criteria were American Society of Anesthesiologists classifications I and II, ages 18–65 years, and a plan for postoperative PCA. The exclusion criteria were a known history of allergy to either ketamine, parecoxib or morphine for treatment of chronic pain, gastrectomy surgery because of peptic ulcer disease, liver and renal impairment, pregnancy, or morbid obesity. Patients were withdrawn from the study if unanticipated adverse events occurred intraoperatively, or if they were admitted to intensive care unit postoperatively.
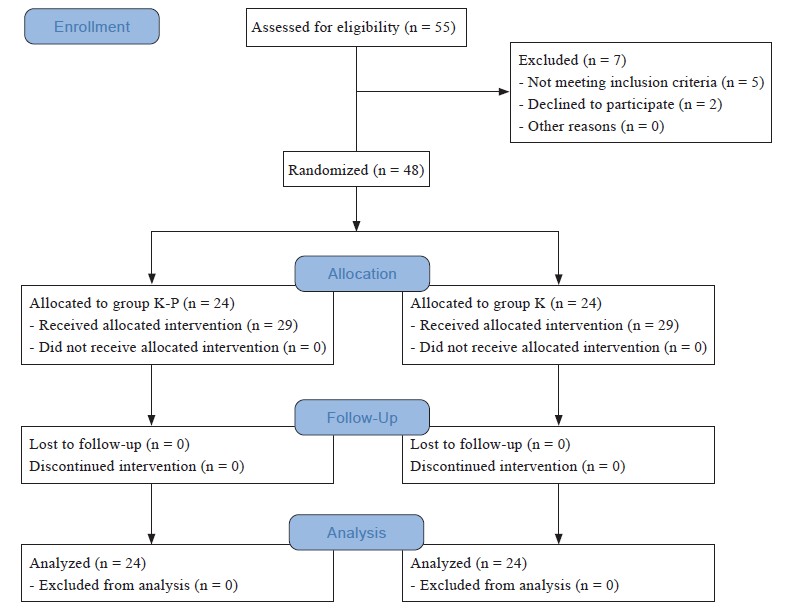
Participants were randomized to two groups of preemptive analgesia by using the computer-generated randomisation. In group K-P (n = 24) a combination of IV ketamine 0.3 mg/kg and IV parecoxib 40.0 mg was given, while, in group K (n = 24), IV ketamine 0.3 mg/kg as placebo was given. Allocation numbers were concealed in a sealed envelope which was only opened on the day of the surgery by the Anaesthesiology Medical Officer in-charge of drug preparation (Figure 1). All patients were premedicated with oral midazolam 7.5 mg on the night prior to surgery and before the operating theatre (OT) call.
Download full-size image
In the OT, an 18 G or 20 G IV cannula was inserted at the hand and non-invasive monitoring devices, such as electrocardiogram, pulse oximetry (SpO2), capnography, and non-invasive blood pressure, were put in place. During pre-oxygenation and within 10 minutes before the induction of anesthesia, patients in group K-P received preemptive analgesia of IV ketamine 0.3 mg/kg and IV parecoxib 40.0 mg in 2 mL of normal saline, while those in group K received IV ketamine 0.3 mg/kg and 2 mL of IV normal saline as placebo. Anesthesia was induced with IV fentanyl 2.0 μg/kg and IV propofol 2.0 mg/kg in all patients, and after successful induction, IV rocuronium 0.6 mg/kg was given as muscle relaxant for endotracheal intubation. After successful intubation, anesthesia was maintained by using sevoflurane, oxygen, and air. All patients were mechanically ventilated to maintain normocapnia with an end-tidal carbon dioxide of 35–40 mmHg. IV morphine 0.1 mg/kg was given for intraoperative analgesia, and an additional bolus of IV fentanyl at 1.0 μg/kg was given as rescue analgesia each time haemodynamic parameters increased to more than 20 % from baseline because of intraoperative pain. Total intraoperative requirement of IV fentanyl and IV morphine were documented. At the end of the surgery, IV ondansetron 4.0 mg was given as prophylaxis against postoperative nausea and vomiting. Muscle relaxant effects were reversed by IV neostigmine 2.5 mg and IV atropine 1.0 mg, and patients were extubated after they met the criteria for extubation. They were monitored in the recovery bay for 1 hour before being discharged to the ward.
Postoperative analgesia was initiated at the recovery bay for all patients identically. The first rescue analgesia with IV fentanyl 0.5 μg/kg bolus was considered at the recovery bay if patients experienced severe pain. An automated PCA infusion device that delivered a 1.0 mg bolus of morphine for every demand with a lock-up time of 5 minutes without background infusion was also initiated in the recovery bay for postoperative analgesia within the next 24 hours. Pain severity was assessed postoperatively by using the VAS at 1 hour, and subsequently at 4, 8, 12, 16, 20, and 24 hours. Time-to-first morphine request recorded by PCA device and total morphine requirement delivered by PCA device over 24 hours were documented.
Sample size was calculated using the Power and Sample Size software, version 3.0 (January 2009, ©1997–2009, Dupont WD and Plummer WD) and was based on a previous study by Behdad et al.,14 in which the response within each group was normally distributed with standard deviation 0.6. Based on an expected difference of 0.5 among between-group means, our study required 24 experimental subjects and 24 control subjects to reject the null hypothesis that the population means of the experimental and control groups were equal with a probability (power) of 0.8. The type I error probability associated with this null hypothesis was set at 0.05.
Data were analyzed by using the Statistical Package for the Social Sciences (SPSS) software, ver. 24.0 (IBM SPSS Inc., Armonk, NY, USA). Categorical data were analyzed by using the chi-square test, while numerical data were analyzed by the independent t-test. P-value < 0.05 was considered statistically significant.
Results
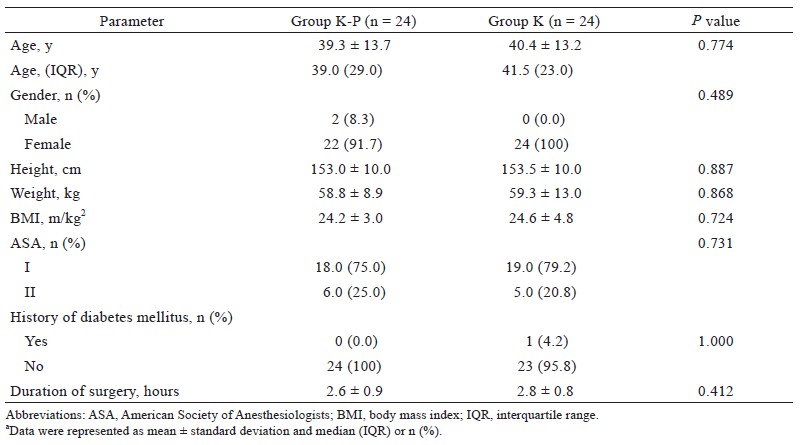
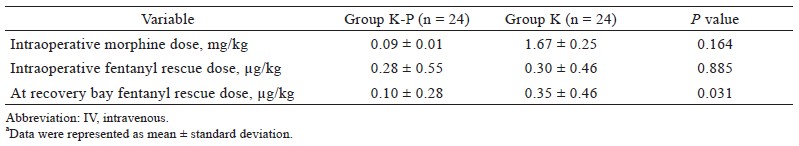
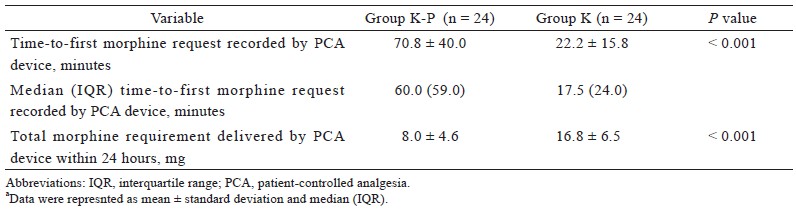
There were no significant differences in demographic characteristics between the two groups (Table 1). Compared to group K, patients in group K-P required significantly lower doses of IV fentanyl (rescue analgesia) in the recovery bay (0.10 ± 0.28 vs. 0.35 ± 0.46 μg/kg; P = 0.031). However, there were no significant differences in intraoperative morphine or fentanyl requirements between the two groups (Table 2). Group K-P also displayed significantly prolonged time-to-first morphine request recorded by PCA device (70.8 ± 40.0 vs. 22.2 ± 15.7 mins; P < 0.001) and lower total morphine requirement delivered by PCA device during the first 24 hours after surgery (8.0 ± 4.6 vs. 16.8 ± 6.5 mg; P < 0.001) (Table 3). In terms of pain intensity, compared to group K, patients in group K-P showed significantly lower VAS values at all time points tested during 24 hours after surgery (Table 4).
Download full-size image
Download full-size image
Download full-size image
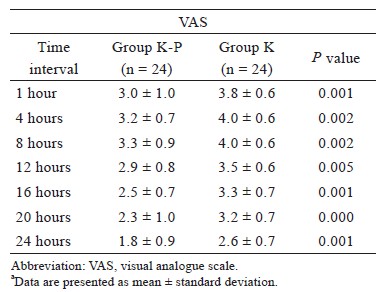
Download full-size image
Discussion
We investigated the utility of multimodal preemptive analgesia by using a combination of IV ketamine and IV parecoxib by comparing it with analgesia using IV ketamine alone. We showed that, compared to IV ketamine alone, a combination of ketamine and parecoxib led to a significantly lower requirement of both rescue analgesia at the recovery bay and morphine delivered by PCA device during 24 hours post-procedure, apart from prolonged time-tofirst morphine request recorded by PCA device, and lower VAS values at all intervals tested. However, there was no significant difference in intraoperative opioid requirement between the two groups.
To the best of our knowledge, no study has compared multimodal preemptive analgesia involving a combination of IV ketamine and IV parecoxib in laparotomy patients. However, one previous study that compared preemptive IV ketamine 0.5 mg/kg, IV parecoxib 40 mg, and placebo in 81 patients undergoing laparoscopic uterine surgery who also received postoperative sufentanil-based PCA showed that a single injection of short-acting ketamine before surgery had the same efficacy as long-acting parecoxib with respect to an opioid-sparing effect in the first 24 hours after surgery. However, it is known that parecoxib has better analgesic effect in the early postoperative period,13 and based on this fact, we show that a combination of IV ketamine and IV parecoxib led to better postoperative analgesia compared to single therapy. Importantly, we used a lower dose of IV ketamine at 0.3 mg/kg while the study on uterine surgery used 0.5 mg/kg.
We reviewed the studies on multimodal preemptive analgesia that used ketamine to be combined with other drugs. One study compared the effectiveness of a combination of IV ketamine 0.15 mg/kg and the non-steroidal anti-inflammatory drug diclofenac at 1.00 mg/kg with that of placebo, ketamine, or diclofenac alone in 80 consecutive patients undergoing laparoscopic cholecystectomy and reported that, at one hour after surgery, patients who received the combination had significantly lower pain scores compared with those receiving placebo or ketamine alone. Further, even though patients from all the four groups required postoperative analgesia, the time-todiclofenac sodium request was significantly longer in patients given the combination compared to those given placebo, ketamine, or diclofenac alone. This study also stated that ketamine at a dose of 0.15 mg/kg did not elicit a preemptive analgesic effect15 but supported the concept of multimodal preemptive analgesia. It is possible that the lower dose of IV ketamine (0.15 mg/kg) used in that study did not yield any significant effect.
There are conflicting results on the efficacy of IV ketamine as preemptive analgesia because while some studies have demonstrated its usefulness, others have shown no beneficial effects. Singh et al.,3 in their study on multiple dosing of IV ketamine, at 1.00, 0.75 and 0.50 mg/kg for laparoscopic cholecystectomy, found that preemptive ketamine had a definitive role in reducing postoperative pain and analgesia requirement. Further, among the three tested doses, 0.5 mg/ kg led to no adverse effects or haemodynamic changes while the dose of 1.00 mg/kg resulted in significantly higher heart rate and blood pressure, compared with other groups, at 0.0 and 0.5 hours, apart from a 10% incidence of hallucinations. Therefore, 0.5 mg/kg was considered the optimal dose for preemptive analgesia in patients undergoing laparoscopic cholecystectomy.3 Another RCT among 80 adult male patients undergoing surgery for acute appendicitis used IV ketamine 0.5 mg/kg 10 minutes before the surgical incision as preemptive analgesia and showed that, compared with the placebo group, the IV ketamine group had a significantly prolonged time-to-first analgesia request (23.1 ± 6.7 vs. 18.1 ± 7.3 minutes; P = 0.02) and required fewer pethidine injections in the first 24 hours after the procedure (0.6 ± 0.6 vs. 2.0 ± 0.80; P = 0.032). Further, there were no drug-related side effects in the ketamine group.14 In contrast to the above, the study by Nistal-Nuño et al.16 used 0.5 mg/kg IV ketamine before surgical incision and did not report a preemptive analgesic effect or a reduction in postoperative opioid requirement for pain in patients given IV morphine after colon surgery. Similarly, two other studies that used low-dose IV ketamine 0.5 mg/kg as preemptive analgesia during elective caesarean sections performed under either spinal anesthesia or general anesthesia showed that low-dose ketamine did not have a preemptive analgesic effect and that it was not effective in reducing postoperative pain scores or opioid requirements.17,18
One study by Heydari et al.19 compared the effectiveness of three preemptive analgesic agents, namely, IV ketamine 0.25 mg/kg, IV paracetamol 15 mg/kg, IV magnesium sulphate 7.5 mg/kg, and placebo. The drugs were administered immediately after the induction of anesthesia in patients scheduled for elective lower extremity orthopaedic surgery under general anesthesia and the results showed that mean postoperative pain score during 24 hours after surgery and mean additive analgesic use during 12 hours after surgery were significantly lower in the ketamine group compared to others. Further, excellent and good satisfaction scores were also significantly more frequent in the ketamine group compared to the other groups.19
The use of parecoxib as preemptive analgesia has been previously studied in a few types of surgery. Xiao et al.20 studied the effect of preemptive IV parecoxib, 40 mg, given 30 minutes before the first incision for total hip arthroplasty, by comparing it with a placebo. In that study, parecoxib was part of a multimodal analgesia regimen with subsequent doses provided every 12 hours for 2 days postoperatively, along with morphine delivered by PCA device, and the results showed that preemptive IV parecoxib was an effective addition to a multimodal regimen that alleviated postoperative pain and reduced cumulative morphine consumption, length of hospitalisation, and perioperative inflammatory response, without increasing perioperative bleeding risk.20 Another study by Bian et al.12 on preemptive IV parecoxib for total knee arthroplasty showed that it significantly decreased VAS values in the short term, relieved pain shortly after surgery, and did not increase the incidence of complications.12 Preemptive analgesia with IV parecoxib has also been shown to relieve acute pain when administered to patients undergoing radical resection for lung cancer and was helpful in reducing the incidence of emergence agitation.21 Additionally, in thyroid carcinoma patients requiring surgery, combined application of IV parecoxib as preemptive analgesia before anesthesia and subsequently after surgery reduced the levels of plasma stress hormones and improved analgesic effects.22
Conclusions
Multimodal preemptive analgesia using a combination of low-dose IV ketamine 0.3 mg/kg and IV parecoxib 40 mg was more effective than IV ketamine 0.3 mg/kg alone in post-laparotomy patients who were provided with morphine by PCA device as postoperative analgesia.
Acknowledgments
Part of the manuscript has been presented in the 3rd International Conference on Medical and Health Sciences—24th National Conference on Medical and Health Sciences (3rd ICMHS & 24th NCMHS) Annual Conference 2019, Kota Bharu, Kelantan, Malaysia.
Conflict of Interest
None.
Financial Disclosures
None.
References
| 1 |
Rosero EB, Joshi GP.
Preemptive, preventive, multimodal
analgesia: what do they really mean?.
Plast Reconstr
Surg. 2014;134(4 Suppl 2):85S-93S.
|
| 2 |
Long JB, Bevil K, Giles DL.
Preemptive analgesia in minimally
invasive gynecologic surgery.
J Minim Invasive Gynecol.
2019;26(2):198-218.
|
| 3 |
Singh H, Kundra S, Singh RM, Grewal A, Kaul TK, Sood
D.
Preemptive analgesia with ketamine for laparoscopic
cholecystectomy.
J Anaesthesiol Clin Pharmacol.
2013;29(4):478-484.
|
| 4 |
Jianda X, Yuxing Q, Yi G, Hong Z, Libo P, Jianning Z.
Impact
of preemptive analgesia on inflammatory responses
and rehabilitation after primary total knee arthroplasty:
a controlled clinical study.
Sci Rep. 2016;6:30354.
|
| 5 |
Aweke Z, Seyoum F, Shitemaw T, Doba DN.
Comparison
of preemptive paracetamol, paracetamol-diclofenac &
paracetamol-tramadol combination on postoperative pain
after elective abdominal surgery under general anesthesia,
Ethiopia: a randomized control trial study, 2018.
BMC
Anesthesiol. 2020;20(1):191.
|
| 6 |
Makkar JK, Jain K, Kuberan A, Balasubramanian M,
Bhatia N, Singh PM.
Pre-emptive multimodal analgesic
regimen reduces post-operative epidural demand boluses
in traumatic shaft of femur fracture—a randomised
controlled trial.
Indian J Anaesth. 2019;63(11):895-899.
|
| 7 |
Savitha KS, Dhanpal R, Kothari AN.
The effect of multimodal
analgesia on intraoperative morphine requirement
in lumbar spine surgeries.
Anesth Essays Res.
2017;11(2):397-400.
|
| 8 |
Simpson JC, Bao X, Agarwala A.
Pain management in enhanced
recovery after surgery (ERAS) protocols.
Clin Colon
Rectal Surg. 2019;32(2):121-128.
|
| 9 |
Yang L, Zhang J, Zhang Z, Zhang C, Zhao D, Li J.
Preemptive
analgesia effects of ketamine in patients undergoing
surgery. A meta-analysis.
Acta Cir Bras. 2014;29(12):819-
825.
|
| 10 |
Van Backer JT, Jordan MR, Leahy DT, et al.
Preemptive
analgesia decreases pain following anorectal surgery: a
prospective, randomized, double-blinded, placebo-controlled
trial.
Dis Colon Rectum. 2018;61(7):824-829.
|
| 11 |
Yang J, Hao Z, Li W, et al.
The efficacy and safety of
paravertebral block combined with parecoxib during video-
assisted thoracic surgery: a randomized controlled trial.
J Pain Res. 2020;13:355-366.
|
| 12 |
Bian YY, Wang LC, Qian WW, et al.
Role of parecoxib
sodium in the multimodal analgesia after total knee arthroplasty:
a randomized double-blinded controlled trial.
Orthop Surg. 2018;10(4):321-327.
|
| 13 |
Liu SM, Yue Y.
The comparison of preemptive analgesic
efficacy between short-acting ketamine and long-acting parecoxib.
Zhonghua Yi Xue Za Zhi. 2018;98(48):3930-
3935. [In
Chinese]
|
| 14 |
Behdad A, Hosseinpour M, Khorasani P.
Preemptive
use of ketamine on post operative pain of appendectomy.
Korean J Pain. 2011;24(3):137-140.
|
| 15 |
Nesek-Adam V, Grizelj-Stojčić E, Mršić V, Rašić Z,
Schwarz D.
Preemptive use of diclofenac in combination
with ketamine in patients undergoing laparoscopic cholecystectomy:
a randomized, double-blind, placebo-controlled
study.
Surg Laparosc Endosc Percutan Tech.
2012;22(3):232-238.
|
| 16 |
Nistal-Nuño B, Freire-Vila E, Castro-Seoane F, Camba-
Rodriguez M.
Preoperative low-dose ketamine has
no preemptive analgesic effect in opioid- naïve patients
undergoing colon surgery when nitrous oxide is used—a
randomized study.
F1000Res. 2014;3:226.
|
| 17 |
Han SY, Jin HC, Yang WD, et al.
The effect of low-dose
ketamine on post-caesarean delivery analgesia after
spinal anesthesia.
Korean J Pain. 2013;26(3):270-276.
|
| 18 |
Reza FM, Zahra F, Esmaeel F, Hossein A.
Preemptive
analgesic effect of ketamine in patients undergoing elective
cesarean section.
Clin J Pain. 2010;26(3):223-226.
|
| 19 |
Heydari SM, Hashemi SJ, Pourali S.
The comparison of
preventive analgesic effects of ketamine, paracetamol and
magnesium sulfate on postoperative pain control in patients
undergoing lower limb surgery: a randomized clinical
trial.
Adv Biomed Res. 2017;6:134.
|
| 20 |
Xiao K, Yu L, Xiao W, et al.
Pain management using
perioperative administration of parecoxib for total hip
arthroplasty: a randomized, double-blind, placebo-controlled
trial.
Pain Physician. 2019;22(6):575-582.
|
| 21 |
Lu J, Liu Z, Xia K, et al.
Effect of preemptive analgesia
with parecoxib sodium in patients undergoing
radical resection of lung cancer.
Int J Clin Exp Med.
2015;8(8):14115-14118.
|
| 22 |
Wang LD, Gao X, Li JY, et al.
Effects of preemptive analgesia
with parecoxib sodium on haemodynamics and
plasma stress hormones in surgical patients with thyroid
carcinoma.
Asian Pac J Cancer Prev. 2015;16(9):3977-
3980.
|