To the Editor,
Postoperative fluid management strategies and fluid balance are important after liver transplantation. Bleeding, drainage of ascites, and insensible water loss may induce hypoperfusion of vital organs such as liver and kidney and further develop organ dysfunction. On the other hand, over-hydration during or after surgery may result in fluid overload with interstitial edema, which also leads to systemic hypoperfusion and impaired tissue oxygenation.1 Many factors, such as low urine output and tachycardia, are triggers for fluid administration. However, low urine output may occur in the first few days after surgery because of the release of antidiuretic hormone as the stress response, and tachycardia may be associated with surgical wound pain. Malbrain et al.2 expound the risk of fluid overload and four phases of fluid management: “resuscitation, optimization, stabilization, and evacuation (ROSE)” in septic shock. The concepts are also conducted in perioperative fluid therapy.3,4 Herein, we share our experience regarding fluid management by using “ROSE” concept in a patient receiving cadaveric liver transplantation.
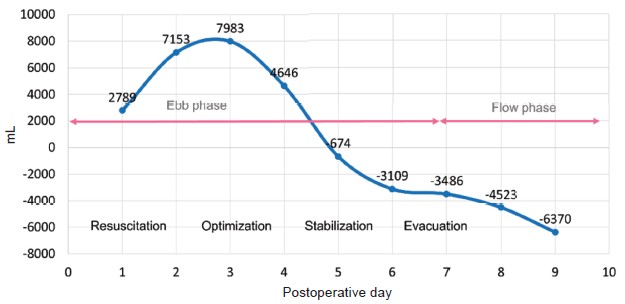
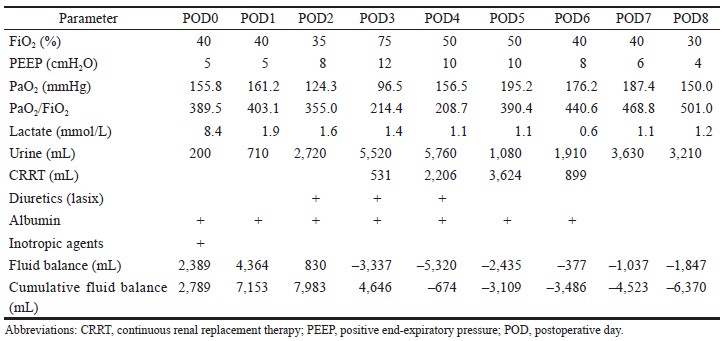
A 57-year-old male (height 169 cm, weight 67 kg) was diagnosed with hepatitis C related hepatocellular carcinoma, cT2N0M0, stage II, status of post-liver trisegmentectomy with recurrence. He received cadaveric liver transplantation under general anesthesia. Total surgical time was about 13 hours. Intraoperative blood loss was 13,650 mL, and urine output was 450 mL. Fluid resuscitation, including crystalloid fluid 14,700 mL, 20% human albumin 250 mL, and blood transfusion with packed red blood cell 40 U, fresh frozen plasma 32 U, and cryoprecipitate 10 U (total 10,310 mL) was administrated based on stroke volume variation and rotational thromboelastometry guidance. Low dose inotropic agent was prescribed to correct shock status at the end of surgery. In intensive care unit, we followed the principles of fluid management recommended by Malbrain et al.2 (Figure 1). Positive fluid balance was achieved in resuscitation and optimization phase (postoperative day [POD] 0–POD 1). In stabilization and evacuation phase (POD 2–POD 6), the lactate level decreased while PaO2/FiO2 ratio worsened. The article written by Malbrain et al.2 suggested that achieving the goal of a zero to negative fluid balance by day 3 and keeping the cumulative fluid balance on day 7 as low as possible by using diuretics or renal replacement therapy after acute resuscitation is reasonable.5 Therefore, we induced a negative fluid balance by using “PAL” treatment (positive end-expiratory pressure [PEEP], albumin, Lasix) and renal replacement therapy.5 Flow phase had been presented since POD 7, and ventilator weaning was carried out on POD 8 (Table 1). The patient was transferred to ordinary ward on POD 9.
Download full-size image
Download full-size image
In conclusion, appropriate timing of fluid administration is important for post-liver transplantation patients especially when the patients present shock status. Although fluid administration provides hemodynamic stabilization initially, the increase in the cumulative fluid balance may cause fluid overload and worsen the outcome. Using four phases (ROSE) concept which is tailored to individual patients during perioperative period may conduce to rationale clarity regarding fluid administration.
Conflict of Interest
None.
Acknowledgement
We thank the patient for signing the informed consent for publication.
References
| 1 |
Kayilioglu SI, Dinc T, Sozen I, Bostanoglu A, Cete M, Coskun F.
Postoperative fluid management.
World J
Crit Care Med. 2015;4(3):192-201.
|
| 2 |
Malbrain MLNG, Van Regenmortel N, Saugel B, et al.
Principles of fl uid management and stewardship in septic
shock: it is time to consider the four D’s and the four
phases of fl uid therapy.
Ann Intensive Care. 2018;8(1):66.
|
| 3 |
Hoste EA, Maitland K, Brudney CS, et al.
Four phases of intravenous fluid therapy: a conceptual model.
Br J Anaesth.
2014;113(5):740-747.
|
| 4 |
Malbrain MLNG, Langer T, Annane D, et al.
Intravenous
fluid therapy in the perioperative and critical care setting:
executive summary of the International Fluid Academy
(IFA).
Ann Intensive Care. 2020;10(1):64.
|
| 5 |
Malbrain MLNG, Marik PE, Witters I, et al.
Fluid
overload, de-resuscitation, and outcomes in critically
ill or injured patients: a systematic review with suggestions
for clinical practice.
Anaesthesiol Intensive Ther.
2014;46(5):361-380.
|