Negative-pressure pulmonary edema (NPPE) is a rare complication that may occur following general anesthesia. In the anesthesia setting, it is typically caused by upper airway obstruction combined with intense inspiratory effort, which generates a strongly negative intrathoracic pressure leading to the increased hydrostatic pressure of pulmonary capillaries and fluid extravasation into the parenchyma and alveoli.1,2 The incidence of NPPE may reach approximately 0.1%, of which extubation-related laryngospasm is the most common cause in anesthetic practice.1 However, most cases of NPPE were reported in the setting of bilateral pulmonary involvement.2 Herein, we attempt to report a patient who developed unilateral NPPE following one-lung ventilation (OLV) for thoracic surgery and share our thoughts regarding the potential mechanism. Informed consent to anonymized reporting of clinical details was obtained from the patient.
A 69-year-old man with an American Society of Anesthesiologists (ASA) physical status III (55 kg; 165 cm; body mass index, 20.2 kg/m2) and a history of hypertension and diabetes mellitus visited our outpatient department of thoracic surgery and presented with dry cough and right-sided chest pain for 3 weeks. His chest X-ray revealed a pulmonary nodule in the right upper lobe of the lung complicated with pleural effusion (Figure 1A). Results of pulmonary function test for forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC were within normal limits. In addition, his routine laboratory workup was unremarkable. Due to the suspicion of malignancy, the patient was admitted and scheduled for thoracoscopic surgery to determine the nature of the pulmonary mass.
In the operation room, general anesthesia was induced with fentanyl (2.0 mcg/kg) and propofol (2.0 mg/kg). After the patient lost consciousness, rocuronium (0.6 mg/kg) was administered to facilitate endobronchial intubation using a left-sided 37-Fr double-lumen tube (DLT; Shiley™ Endobronchial Tube, Mallinckrodt Medical, Athlone, Ireland). The position of the DLT was adjusted by fiberoptic bronchoscopy. Anesthesia was maintained with oxygen in the air, sevoflurane, and fentanyl. Mechanical ventilation was initiated in the volume control model with a tidal volume (VT) of 450 mL at the rate of 10 breaths/min. Then, the patient was turned to the left lateral decubitus position, and correct tube placement was confirmed again. Ten minutes before surgery, OLV was started. During OLV, the patient was ventilated with a VT of 300 mL plus a positive end-expiratory pressure (PEEP) of 5 cmH2O and a peak airway pressure < 35 cmH2O. The level of end-tidal carbon dioxide was maintained at 35–45 mmHg by adjusting the ventilation rate. The 60-min single-port thoracoscopic procedure with wedge resection under general anesthesia with OLV proceeded uneventfully. A stepwise recruitment maneuver was applied to reaerate the collapsed lung when the surgeon started to close the chest wall.
During the period of emergency, the patient was extubated in the supine position after suctioning secretions was performed in the trachea and oropharynx when he regained consciousness and adequate spontaneous ventilation following sugammadex administration (2.0 mg/kg). However, shortly after extubation, he became agitated and began to choke with intermittent stridor. Besides, marked respiratory distress with decreased oxygen saturation at 60% was noted. As laryngospasm was suspected, we promptly performed a laryngospasm notch pressure and applied a jaw thrust maneuver with positive-pressure mask ventilation using 100% oxygen at 10 L/min, but oxygen saturation remained at around 85%. Meanwhile, a great amount of pink frothy sputum was expelled. Chest auscultation revealed coarse inspiratory rhonchi, prominently over the right lung. Due to persistent respiratory distress, the patient was reintubated and transferred to the intensive care unit for further care. A portable chest radiograph showed diffuse pulmonary infiltration over the right lung field (Figure 1B), and diagnostic bronchoscopy found abundant pinkish secretions originating from the right lung. Based on the aforementioned findings, the diagnosis of unilateral NPPE was established. Under positive-pressure mechanical ventilation and diuretic therapy, the patient was extubated 2 days after surgery, and his chest radiograph revealed complete remission of pulmonary infiltration on postoperative day 5.
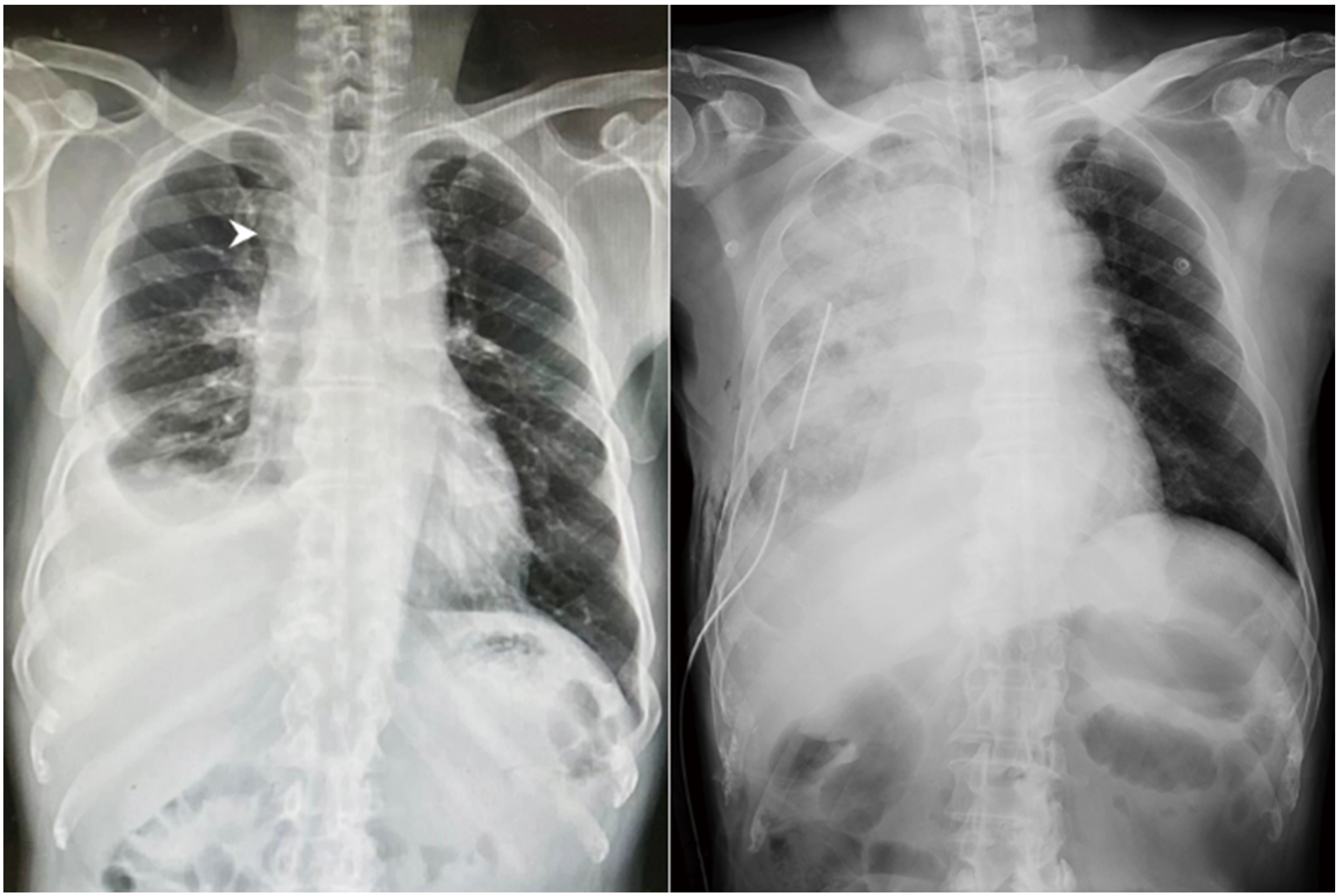
Download full-size image
(A) Preoperative chest radiograph showed one solitary pulmonary nodule in the right upper lobe of the lung (white arrowhead) with right-sided pleural effusion. (B) A portable chest radiograph revealed diffuse pulmonary infiltration of the right lung field.
In this case, clinical presentations shortly after extubation strongly implied the incidence of postoperative laryngospasm, which manifests as a protective reflex of the upper airway. The muscular contraction in the episode of laryngospasm usually responds to intrinsically mechanical or chemical stimuli, and extrinsically painful stimuli.1 Sugammadex, a selective agent that can reverse rocuronium-induced neuromuscular blockade, has also been reported to be associated with the occurrence of NPPE because of the rapid and complete return of respiratory muscle tone during acute upper airway obstruction.3 In addition, sugammadex itself may lead to laryngospasm as in our previous report,4 which can contribute to NPPE. However, our patient presented with asymmetric pulmonary infiltration, which was different from the typical features of NPPE.
Unilateral PE is mainly reported in cases with heart disease, prolonged lateral decubitus position, and lung re-expansion after fluid or air was rapidly drained from the pleural space.5 Our patient suffered from diffuse pulmonary infiltration mostly in the non-dependent lung, which did not seem to be related to prolonged lateral decubitus position and fluid overload. Moreover, the right lung was fully expanded by stepwise lung recruitment at the end of the surgery, and the chest tube was put to a water seal rather than connected to a negative-pressure suction system. Nevertheless, surgical manipulation and re-expansion of the collapsed lung invariably induce lung injury.6-8 Meanwhile, inflammatory cytokines are released in response to localized injury and may also promote more extensive lung injury.6 Accordingly, surgical trauma and OLV may be associated with unilateral NPPE.
The pathophysiologic mechanisms resulting in acute lung injury after OLV are complex and multifactorial. In the ventilated lung, causative factors include excessive lung volumes, hyperperfusion, and oxidative injury; on the other hand, the collapsed lung is exposed to ischemia-reperfusion injury and recruitment-induced shear stress.6 These harmful effects attributed to OLV can cause overexpression of inflammatory mediators and activation of neutrophils, which may induce diffuse alveolar damage and augment microvascular permeability.6-8 Hence, protective ventilation strategies during OLV should be routinely performed to reduce the extent of the injury. Although there is a lack of standardized practice guidelines for lung protection during OLV, the most commonly recommended strategies during OLV consist of a low VT (4–6 mL/kg predicted body weight), a PEEP (5–10 cmH2O), and cycling recruitment maneuvers with stepwise increases in peak airway pressure and PEEP.6,7 Notably, volatile anesthesia appears to lessen the degree of lung injury during OLV.6 However, in addition to ischemia-reperfusion injury and lung recruitment, the level of surgical trauma is a significant precipitating factor of acute lung injury after OLV,6-8 which may make the collapsed lung vulnerable to disorders with increased pulmonary capillary pressure as our case despite performing recommended lung-protective strategies reported previously.
Generally, regardless of unilateral or bilateral pulmonary involvement in patients developing NPPE, the standard therapeutic strategies are consistent. Treatment of NPPE should include immediately relieving upper airway obstruction, instituting lung-protective positive-pressure ventilation with supplemental oxygen, and reducing pulmonary vascular pressure by diuretics, which usually lead to the resolution of PE and respiratory failure within 48 hours.2
This case emphasizes that the occurrence of upper airway obstruction such as laryngospasm may increase the risk of unilateral NPPE for patients receiving OLV for thoracic surgery. There are some topics that we can learn to avoid and manage unilateral NPPE in a similar scenario. First, anesthesiologists should proactively prevent laryngospasm during an emergency and treat it promptly once upper airway obstruction exists. Second, protective ventilation during OLV including low VT ventilation, PEEP application, and stepwise alveolar recruitment should be used in an attempt to minimize lung injury. Additionally, limiting the degree of surgical manipulation is a crucial way to reduce diffuse alveolar damage in the collapsed lung. Last, when patients suffer from NPPE, rapid recognition and adequate treatment are key factors to lower the incidence of morbidity and mortality.
Conflict of Interest
The authors declare no potential conflicts of interest for the research, authorship, and publication of this article.
Funding
The authors receive no financial support for the research, authorship, and publication of this article.
References
| 1 |
Deepika K, Kenaan CA, Barrocas AM, Fonseca JJ, Bikazi GB.
Negative pressure pulmonary edema after acute upper airway obstruction.
J Clin Anesth. 1997;9(5):403-408.
|
| 2 |
Bhattacharya M, Kallet RH, Ware LB, Matthay MA.
Negative-pressure pulmonary edema.
Chest. 2016;150(4):927-933.
|
| 3 |
Kao CL, Kuo CY, Su YK, Hung KC.
Incidence of negative-pressure pulmonary edema following sugammadex administration during anesthesia emergence: a pilot audit of 27,498 general anesthesia patients and literature review.
J Clin Anesth. 2020;62:109728.
|
| 4 |
Wu TS, Tseng WC, Lai HC, Huang YH, Wu ZF.
Sugammadex and laryngospasm.
J Clin Anesth. 2019;56:52.
|
| 5 |
Calenoff L, Kruglik GD, Woodruff A.
Unilateral pulmonary edema.
Radiology. 1978;126(1):19-24.
|
| 6 |
Lohser J, Slinger P.
Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung.
Anesth Analg. 2015;121(2):302-318.
|
| 7 |
Bernasconi F, Piccioni F.
One-lung ventilation for thoracic surgery: current perspectives.
Tumori. 2017;103(6):495-503.
|
| 8 |
Gothard J.
Lung injury after thoracic surgery and one-lung ventilation.
Curr Opin Anaesthesiol. 2006;19(1):5-10.
|