Abstract
Background
The NALDEBAIN® has been available since 2017, and high incidence of injection reactions in the phase 3 study has been reported. Since the first year in the market, the injection site reactions were still the majority of adverse drug reactions (ADRs) in pharmacovigilance reports. The new intramuscular (IM) instruction and package was introduced in the middle of 2018. In this retrospective study, we analyzed the pharmacovigilance data and published postmarketing studies to investigate the impact of IM injection-related reactions in Taiwan between the period of 2017–2022.
Methods
Individual case safety reports (ICSRs) and ADRs were classified by system organ class and preferred term. The reporting rate of ICSRs was used to evaluate the impact of the new IM instruction and package.
Results
A total of 37 ICSRs were identified from pharmacovigilance reports. Among them, 51% of IM injection-related reactions were reported after one single dose of NALDEBAIN administration. The reporting rate of IM injection-related reactions in pharmacovigilance data dropped from 125.00 to 3.56 per ten thousand exposures after IM instruction and package revision in 2018. In addition, the percentage of IM injection-related reactions also reduced in postmarketing studies from 27.5% to 4.5%. There were no serious IM injection-related reactions found in the pharmacovigilance and postmarketing dataset.
Conclusion
Injection site reactions were common after intramuscularly administered oil-based agents during the first year which is later markedly reduced by changing the length of the needle and injection education.
Keywords
injection site reactions, intramuscular injection, nalbuphine
Introductıon
NALDEBAIN® (150 mg dinalbuphine sebacate) is a pro-drug of nalbuphine, which acts as a mixed kappa opioid agonist/mu opioid antagonist providing sufficient analgesia for moderate to severe pain after a single intramuscular (IM) administration in Taiwan and South Asia countries. The product is available as an oil formulation for injection and is supplied in a single-use vial containing 150 mg of dinalbuphine sebacate in 2 mL. Dinalbuphine sebacate can rapidly hydrolyze to nalbuphine by esterases in the human bloodstream.1 Clinical studies had reported that preoperative IM administration of NALDEBAIN exhibits a well-tolerated safety profile and weak analgesic effect, and required less postoperative analgesic use in several different surgery types of patients.2-5
Injection site reactions were major complications for injectable agents, with the incidence rate ranging from 0.5% to 40%, and 80% with oil-based depot injection.6,7 A high incidence of injection site reactions showed in phase 3 of NALDEBAIN study (27.5%).8 All cases were administered to the gluteus maximus by health professionals in the clinical trial. However, IM injection-related reactions remained the majority of postmarketing spontaneous reporting cases.
Since the middle of 2018, we conducted new IM instruction including step-by-step procedures and video-assisted training courses. The medicine was recommended to be administered to the upper outer quadrant of the buttocks, gluteus medius, by Z-track technique to prevent backflow while the needle was withdrawn. Ultrasound-guided IM injection was also suggested, especially for patients with body mass index (BMI) > 25.9 Also, the revision of the commercial package contained one 22 gauge, 1.5-inch length of safety needle and one 2 mL vial with dinalbuphine sebacate solution.10 Adequate length of needle along with well-designed IM injection technique might improve the success rate of IM injection and minimize the occurrence of IM injection-related reactions. Therefore, we analyzed data on spontaneous pharmacovigilance reports and published investigator-initiated studies in Taiwan to evaluate the effect of the new IM injection guide on IM injection-related reactions.
Methods
Study Design
This retrospective descriptive study was based on selected individual case safety reports (ICSRs) from Lumosa Therapeutics reporting to Taiwan Food and Drug Administration and the safety information in the published literature.
We collected the postmarketing spontaneous reporting ICSRs from March 2, 2017 to March 2, 2022. The literature search was conducted through PubMed which was published between January 1, 2017, and June 2, 2022. Those published articles with case report and review format were excluded. All identified ICSRs and adverse drug reactions (ADRs) were carried out by system organ class and preferred term. The reporting rate of IM injection-related reactions was further analyzed by pharmacovigilance reporting period: 6 months for the first two years and 12 months for further three years.
Statistical Analysis
The ICSR and safety profile from clinical trials were summarized by descriptive analysis using Microsoft Office Excel. The calculation of ICSR reporting rate was the number of ICSR divided by estimated NALDEBAIN exposure in the respective period and multiplied by 10,000.
Results
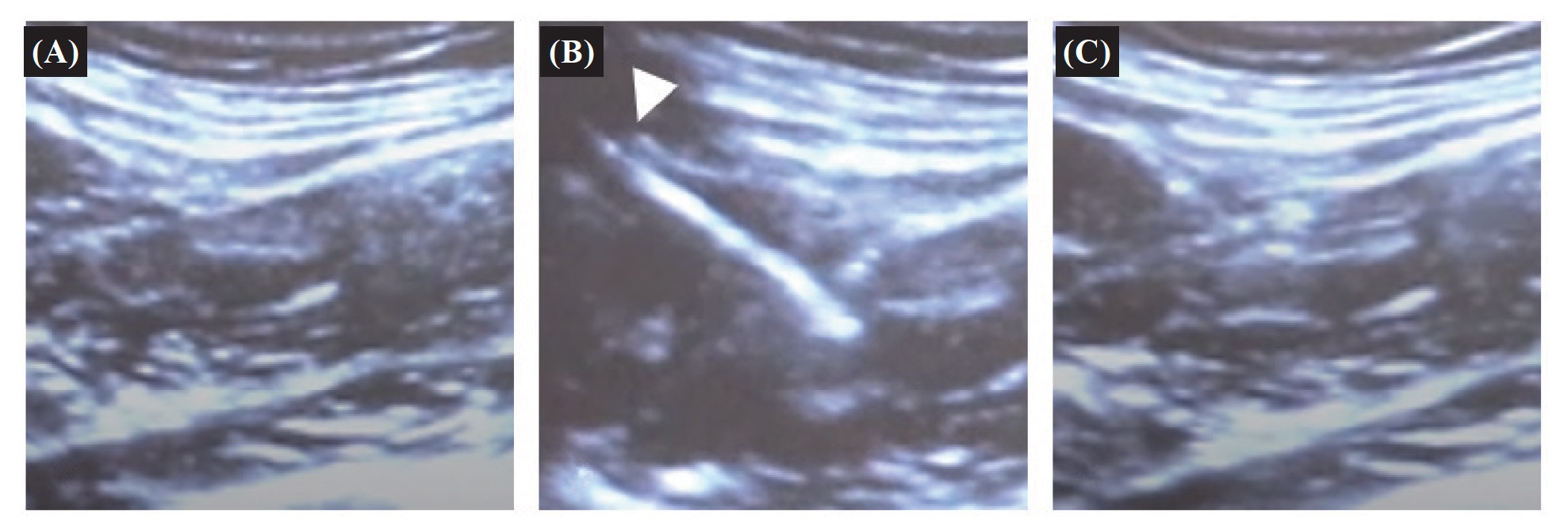
The original and updated version of IM training instruction and the content in the package box is shown in Table 1. The ultrasound-guided IM injection with one 22 gauge, 1.5-inch length of safety needle by Z track technique is presented in Figure 1.

Download full-size image
Download full-size image
(A) Before IM injection; (B) View of gluteus medius muscle by the insertion of the needle (white arrowhead); (C) After medicine administration by Z-track technique.
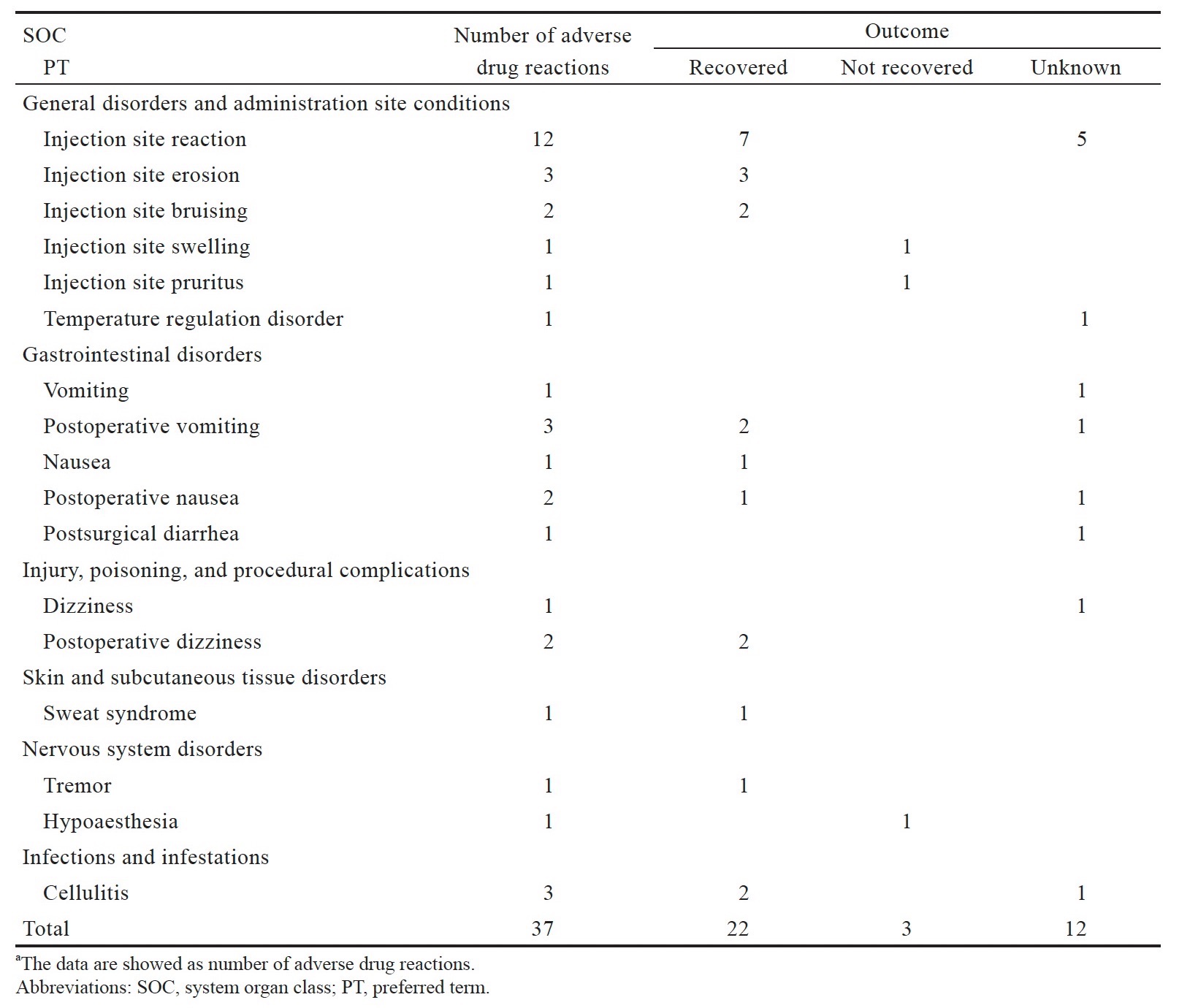
In the NALDEBAIN pharmacovigilance database, we identified 37 ICSRs for NALDEBAIN from 2017 up to 2 March 2022. All selected reported terms are listed together with their frequency of reaction in Table 2. The most frequently reported term was injection site reaction (n = 12) without further specification, followed by injection site erosion, postoperative vomiting, and cellulitis (n = 3). The majority of the reactions were IM injection-related reactions (n = 19), including injection site erosion, bruising, swelling, and pruritus. The majority of the reactions recovered (n = 22); for 12 reactions the outcome was unknown; for 3 reactions was not recovered up to the latest follow-up. No serious ICSR from spontaneous reports was reported from March 2017 to March 2022.
Download full-size image
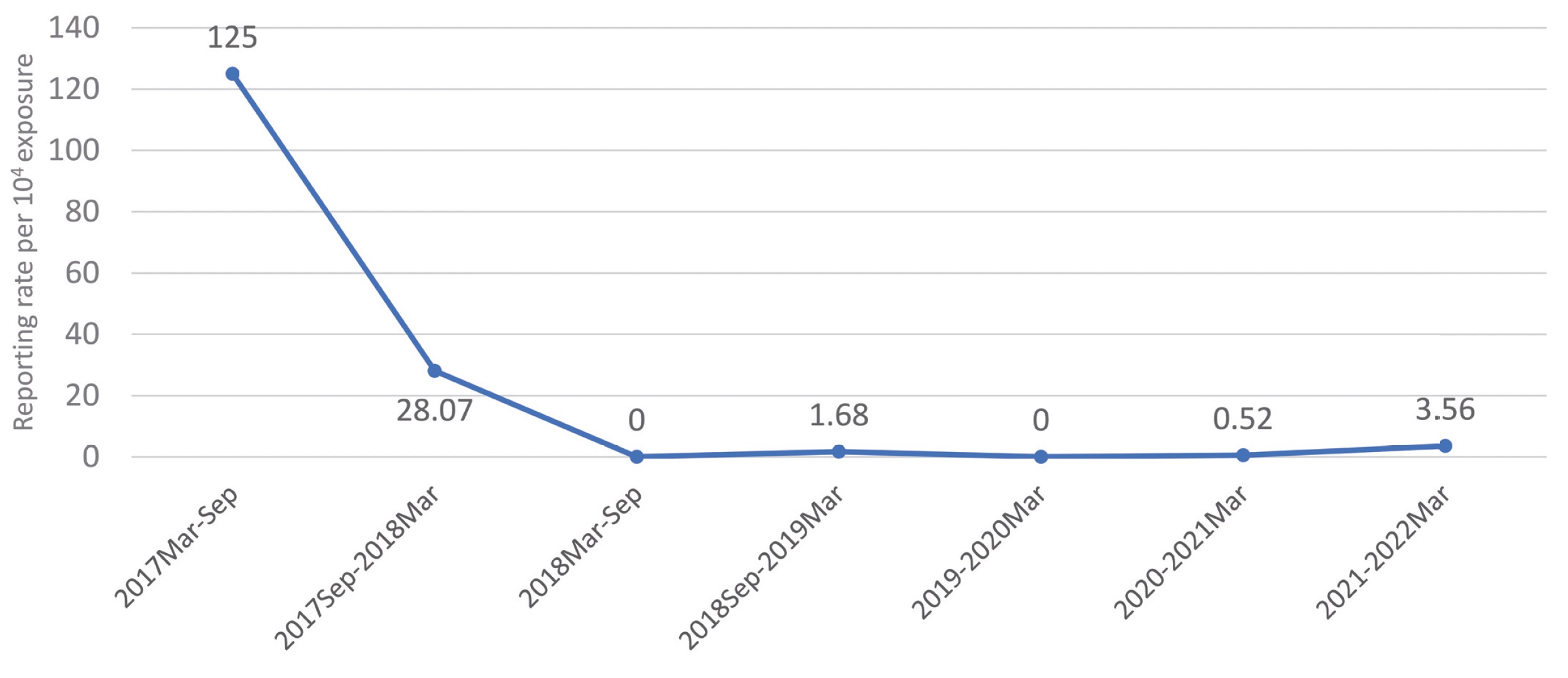
The reporting rate of IM injection-related reactions per ten thousand exposures was shown in Figure 2. Before September 2018, the reporting rate of IM injection-related reactions was at a high level, ranging from 28.07 to 125.00 cases per ten thousand exposures. After September 2018, IM instruction and package updated, the reporting rate of IM injection-related reactions turned down toward the lower level, 3.56 cases per ten thousand or less. The decrease of the reporting rate of IM injection-related reactions was 86% to 97%.
Download full-size image
IM injection-related reactions included injection site reactions, injection site swelling, erosion, bruising, and pruritus. The reporting rate was calculated the number of ICSR divided by estimated NALDEBAIN exposure in the respective period and multiplied by 10,000. The exposures were 400; 1,781; 5,214; 5,945; 15,410; 19,297; 19,644 in the period 2017–2022, respectively.
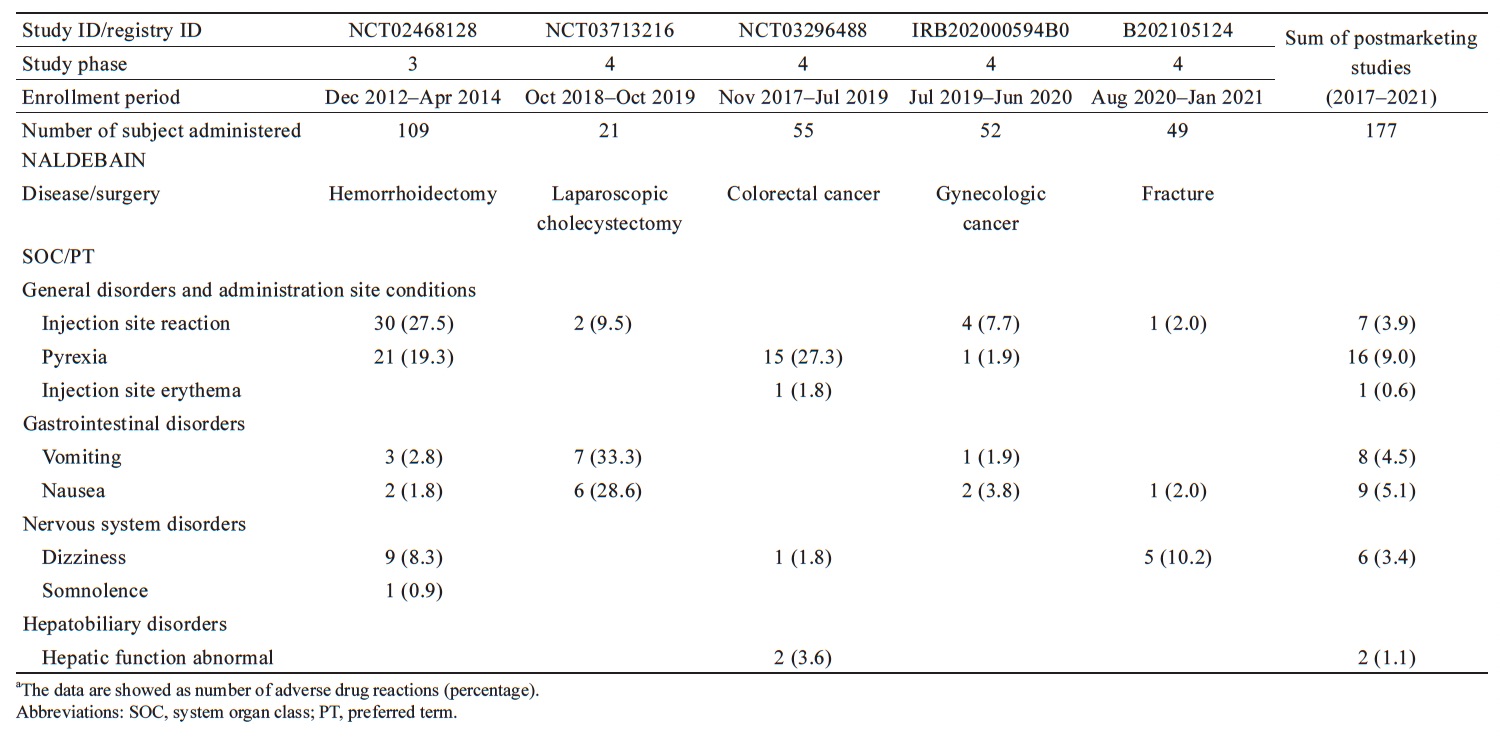
In published NALDEBAIN clinical trials, all reported ADRs are summarized in Table 3. The most frequently reported term was injection site reaction (n = 30, 27.5%) in the hemorrhoidectomy study, vomiting (n = 7, 33.3%) in cholecystectomy study, pyrexia (n = 15, 27.3%) in colorectal cancer population, injection site reaction (n = 4, 7.7%) in the gynecologic cancer population, and dizziness (n = 5, 10.2%) in the fracture study. The majority of ADRs were injection site reactions in the phase 3 study (hemorrhoidectomy), but few injection site reactions were observed in the postmarketing clinical trials. The average incidence of IM injection-related reactions was 4.5% in pooled postmarketing studies. The decrease of incidence of IM injection-related reactions was 80%.
Download full-size image
Dıscussıon
In this retrospective study, both pharmacovigilance and postmarketing clinical data regarding IM injection-related reactions caused by NALDEBAIN administration significantly decreased over 80% after new IM instruction and package updates. Video-assisted IM training and adequate length of needle improved the success rate of IM injection and prevented IM failure due to insufficient length of needle.
The success rate of an IM injection was needed to consider multiple variables, including the injection site anatomy, the injection technique used, the training for the practitioner, and the length of the needle chosen.11 Boyd et al.11 observed only 52% of patients had received a successful IM injection, and the possible reason was insufficient injection depth. In our analysis, one possible reason for a high incidence of injection site reactions during the phase 3 study period was no specific size of needle provided in the package of the investigational drug. Also, video-assisted IM training has better acceptance than step-by-step IM procedures in paper materials for the medical practitioner. The IM injection success rate has greatly improved after the revision of IM instruction and package, evaluating the safety profile of postmarketing studies and the reporting rate of pharmacovigilance data.
The incidence of IM injection-related reactions in the present analysis was similar to that found in previous studies of long-acting injection by IM administration. Marberger et al.12 noted 18.1% of IM injection-related adverse events (AEs) in prostate cancer patients with sustained-release leuprolide acetate injection. Citrome13 reported injection site reactions rate ranging from 4% to 10% in paliperidone palmitate studies. Thus, the average incidence of 4.5% (0.0%–9.5%) IM injection-related AEs observed in postmarketing NALDEBAIN studies appears within the same range as that observed with other IM long-acting injections.
In previous studies, the significant correlation between BMI and subcutaneous fat thickness for the dorsogluteal and ventrogluteal site has been confirmed.14,15 Zaybak et al.16 recommended that ultrasound measurement should be needed in patients with BMI higher than 25 kg/m2. We recommended monitoring by ultrasound during IM injection, especially for obese patients. Visible ultrasonography can help medical practitioners evaluate and confirm the thickness of the subcutaneous tissue and muscle, where the probe is held at a 90° angle to the direction of the injection site.
There are some limitations to this study. First, insufficient data on BMI for postmarketing ICSR to further verify the relationship between each IM-related reaction case. In addition, the small size of the pharmacovigilance database observed a significant change in the reporting rate of IM injection-related reactions, but it might need to follow up on the trend while accumulating a larger number of ICSR cases. Last, even though ultrasound-guided IM injection provided a clear anatomical image under the injection site and verified the administration of medicine into the muscle, it was not a mandatory procedure due to limited equipment in the injection room of each clinic and hospital.
Conclusion
Based on this study, routine IM injection training for the medical practitioner and the adequate length of the needle are the most critical factors to improve the IM injection success rate and prevent IM injection-related reactions.
Acknowledgments
NALDEBAIN® postmarketing spontaneous reporting ICSRs were provided by Lumosa Therapeutics. Co., Ltd.
Author Contributions
KMM wrote the manuscript. WZS and CSW analyzed the data and approved the article. SOL and CHL reviewed the manuscript and provided critical opinions.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
| 1 |
Tien YE, Huang WC, Kuo HY, et al.
Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after
intramuscular injection of dinalbuphine sebacate in an extended-release
formulation.
Biopharm Drug Dispos. 2017;38(8):494-497.
|
| 2 |
Chang TK, Huang CW, Su WC, et al.
Extended-release dinalbuphine sebacate versus intravenous patient-controlled
analgesia with fentanyl for postoperative moderate-to-severe pain: a randomized
controlled trial.
Pain Ther. 2020;9(2):671-681.
|
| 3 |
Lee SO, Huang LP, Wong CS.
Preoperative administration of extended-release dinalbuphine sebacate compares
with morphine for post-laparoscopic cholecystectomy pain management: a
randomized study.
J Pain Res. 2020;13:2247-2253.
|
| 4 |
Chang SH, Chang TC, Chen MY, Chen WC, Chou HH.
Comparison of the efficacy and safety of dinalbuphine sebacate,
patient-controlled analgesia, and conventional analgesia after laparotomy for
gynecologic cancers: a retrospective study.
J Pain Res. 2021;14:1763-1771.
|
| 5 |
Zheng ZH, Yeh TT, Yeh CC, et al.
Multimodal analgesia with extended-release dinalbuphine sebacate for
perioperative pain management in upper extremity trauma surgery: a retrospective
comparative study.
Pain Ther. 2022;11(2):643-653.
|
| 6 |
Thomaidou E, Ramot Y.
Injection site reactions with the use of biological agents.
Dermatol Ther. 2019;32(2):e12817.
|
| 7 |
Sartorius G, Fennell C, Spasevska S, Turner L, Conway AJ, Handelsman DJ.
Factors influencing time course of pain after depot oil intramuscular injection
of testosterone undecanoate.
Asian J Androl. 2010;12(2):227-233.
|
| 8 |
Yeh CY, Jao SW, Chen JS, et al.
Sebacoyl dinalbuphine ester extended-release injection for long-acting
analgesia: a multicenter, randomized, double-blind, and placebo-controlled study
in hemorrhoidectomy patients.
Clin J Pain. 2017;33(5):429-434.
|
| 9 |
Marshall HS, Clarke MF, Evans S, Piotto L, Gent RJ.
Randomized trial using ultrasound to assess intramuscular vaccination at a 60°
or 90° needle angle.
Vaccine. 2013;31(23):2647-2652.
|
| 10 |
Tanioka T, Takase K, Yasuhara Y, et al.
Efficacy and safety in intramuscular injection techniques using
ultrasonographic data.
Health (N Y). 2018;10(3):334-350.
|
| 11 |
Boyd AE, DeFord LL, Mares JE, et al.
Improving the success rate of gluteal intramuscular injections.
Pancreas. 2013;42(5):878-882.
|
| 12 |
Marberger M, Kaisary AV, Shore ND, et al.
Effectiveness, pharmacokinetics, and safety of a new sustained-release
leuprolide acetate 3.
Clin Ther. 2010;32(4):744-757.
|
| 13 |
Citrome L.
Paliperidone palmitate—review of the efficacy, safety and cost of a new
second-generation depot antipsychotic medication.
Int J Clin Pract. 2010;64(2):216-239.
|
| 14 |
Chan VO, Colville J, Persaud T, Buckley O, Hamilton S, Torreggiani WC.
Intramuscular injections into the buttocks: are they truly intramuscular?
Eur J Radiol. 2006;58(3):480-484.
|
| 15 |
Kaya N, Salmaslıoğlu A, Terzi B, Turan N, Acunaş B.
The reliability of site determination methods in ventrogluteal area injection:
a cross-sectional study.
Int J Nurs Stud. 2015;52(1):355-360.
|
| 16 |
Zaybak A, Güneş UY, Tamsel S, Khorshid L, Eşer I.
Does obesity prevent the needle from reaching muscle in intramuscular
injections?
J Adv Nurs. 2007;58(6):552-556.
|