Abstract
Background
Preoperative fasting is a common practice to decrease perioperative aspiration risk. The American Society of Anesthesiologists (ASA) recommends preoperative fasting of 8 hours after a full meal. ASA preoperative fasting recommendation is based on the Western diet. A typical Western diet has a higher fat content than Asian standard solid meals. This study aimed to analyze intragastric volume with ultrasound after 6-hour and 8-hour fasting after an Asian traditional solid meal.
Methods
This cohort study recruited 37 subjects from January to February 2019. Subjects were patients scheduled for elective non-digestive surgery and planned for preoperative fasting of 8 hours. Before preoperative fasting, all subjects consumed standard Asian meals. We performed an ultrasound of the gastric antrum during the relaxation phase after two contractions. After a good image was acquired, the cross-sectional area and gastric volume (GV) were calculated. GV was grouped based on a border value of 1.5 mL/kg.
Results
GV 6 hours after solid intake was 30.93 (1.60–205.25) mL, and GV 8 hours after solid intake was 16.34 (0.73–62.49) mL (
Conclusion
Intragastric volume 8 hours after a standard Asian meal intake was lower than 6 hours after a traditional Asian meal.
Keywords
gastric volume, perioperative fasting, ultrasonography
Introduction
Perioperative aspiration is associated with significant morbidity and mortality as it increases the length of hospitalization and mechanical ventilation. Despite the low incidence (< 0.1% –19.0%), the mortality rate of perioperative aspiration depends on the patient and the type of surgery.1 The volume and the type of gastric content are the main determinants of complications severity. The factors of the patients are diabetes, critically ill, digestive, and other conditions that prolong the duration of gastric emptying.1,2 On the contrary, prolonged fasting increases the risk of dehydration, hypoglycemia, and other problems in patients with metabolic comorbidities.1,2
The American Society of Anesthesiologists (ASA) recommends that patients undergo elective surgery fast for 8 hours after consuming fatty or fried foods and 6 hours after consuming light meals or snacks.2 To reduce prolonged fasting, the Indonesian Society of Anesthesiologists and Intensive Care suggests fasting for 6 hours after a full meal. The difference is mainly because the ASA’s preoperative fasting recommendation is based on a Western diet, which differs from the typical Southeast Asian diet. Southeast Asian food primarily consists of carbohydrates in the form of steamed rice and has less fat content. The higher fat content in Western diets is a factor that prolongs gastric emptying.
Ultrasound is a non-invasive and relatively easy examination to provide real-time gastric volume (GV) assessment to predict aspiration risk. However, gastric residual volume resulting in an increased risk of aspiration remains controversial. Healthy fasting patients often have a residual GV of about 1.5 mL/kg without an increased risk of aspiration.1,2 In an emergency, ultrasound helps assess the patient with an unclear fasting condition requiring immediate medical treatment. Gastric ultrasound has become an option in many countries to determine the risk of perioperative aspiration.
This observational cohort study aimed to compare the difference in elective surgery patients’ GV after 6-hour and 8-hour fasting. If there is no difference in both durations of fasting, then a 6-hour fasting period is considered sufficient to reduce the risk of aspiration in elective surgery patients.
Methods
This observational, experimental study involved patients who underwent non-digestive elective surgery from January to February 2019. Thirty-seven subjects were recruited after the Faculty of Medicine Ethics Committee, Universitas Indonesia (No. 1098/UN2-F1/ETIK/2018) approved this study, registered in clinicaltrial.gov (NCT04850976).
We recruited the subjects using a consecutive sampling of patients aged 18–60, with body mass index between 20–30 kg/m2, ASA physical status I–II, and scheduled for elective non-digestive surgery. The subjects were not recruited if they had specific medical conditions such as diabetes mellitus, pregnancy, abdominal distension, intestinal motility disorders, and history of dyspepsia; the subjects did not receive or consume the standard hospital diet; surgery schedule changes in which the subjects were unable to do 8-hour fasting before surgery; and subjects who had an emergency in the perioperative period.
The sample size was calculated using the formula for comparing two means. The confidence interval (CI) and the power were 95% and 80%, respectively. We did a pre-sampling using the same protocols of 6 subjects before the study and found a mean GV of 22.31 ± 18.63 mL. We set the expected mean difference at 10 mL. The calculated sample size was 32 subjects; another 10% was added to anticipate dropouts, resulting in a minimal sample requirement of 35 subjects.
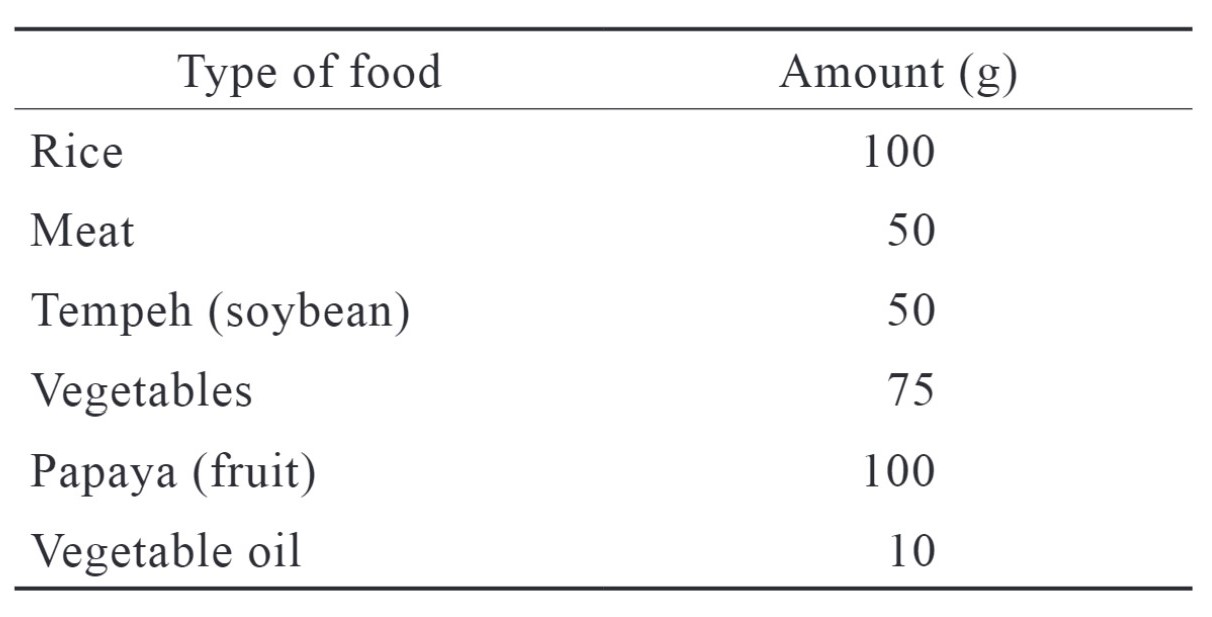
The base data acquired were subject demographic data, including age, weight and height, type of surgery, and preoperative examinations. Subjects were given hospital-standard food with standardized nutritional levels one hour before preoperative fasting started. They could consume clear fluid until two hours before the scheduled surgery. The menu consisted of carbohydrates in the form of rice (64%), proteins (11%), and fat (25%). The menu composition is shown in Table 1.
Download full-size image
Gastric Ultrasound
In this study, we did the gastric ultrasound examination on the subjects in the right lateral decubitus position by using a low-frequency (2–5 MHz) transducer (C5-2s), Mindray unit M7 in the epigastrium region perpendicular to the longitudinal axis of the body. The axial view acquired with this technique enables us to assess the antrum and the gastric wall anatomy. In the empty state, the antrum is visualized as a deflated structure with the anterior and posterior walls sticking together. We measured the diameter of the antrum in the relaxation phase between two peristaltic contractions.3 The diameter was measured as the craniocaudal (CC) and the anteroposterior (AP) diameter. The CC diameter was measured from the most cranial to the most caudal submucosal layer. The AP diameter was measured from the anterior to the most posterior submucosal layer.2 From both diameters, we can calculate the cross-sectional area (CSA) of the antrum using the formula CSA = (π × CC × AP)/4
from Perlas et al.4 We can measure the GV with the formula using the CSA and the age of the subjects [GV = 27.0 + 14.6 × CSA – 1.28 × age (years)]. We grouped the GV into sufficient and insufficient, with a border value of 1.5 mL/kg body weight (BW).4
We did the first gastric ultrasound assessment to record the basal state before and after a meal. We did the ultrasound again in the sixth and eighth hours afterward. We saved all images captured during measurements, and other allocation-blinded research assistants assessed all the measures. The initial results were then compared with the second measurement. Both the people who obtained the data were using the initial measurement, and the images captured were anesthesiologists and anesthesia residents. A regional anesthesiologist consultant trained them in gastric ultrasound examinations before this study. The final CSA and GV were calculated as a mean from both measurements.
The data collected is processed using the Statistical Package for Social Scientists program (SPSS) version 23.0 (IBM Corp., Armonk, NY, USA). Numerical data were tested for normal distribution with the Kolmogorov-Smirnov test, and if the data were normally distributed, the primary outcome was analyzed using the paired
Results
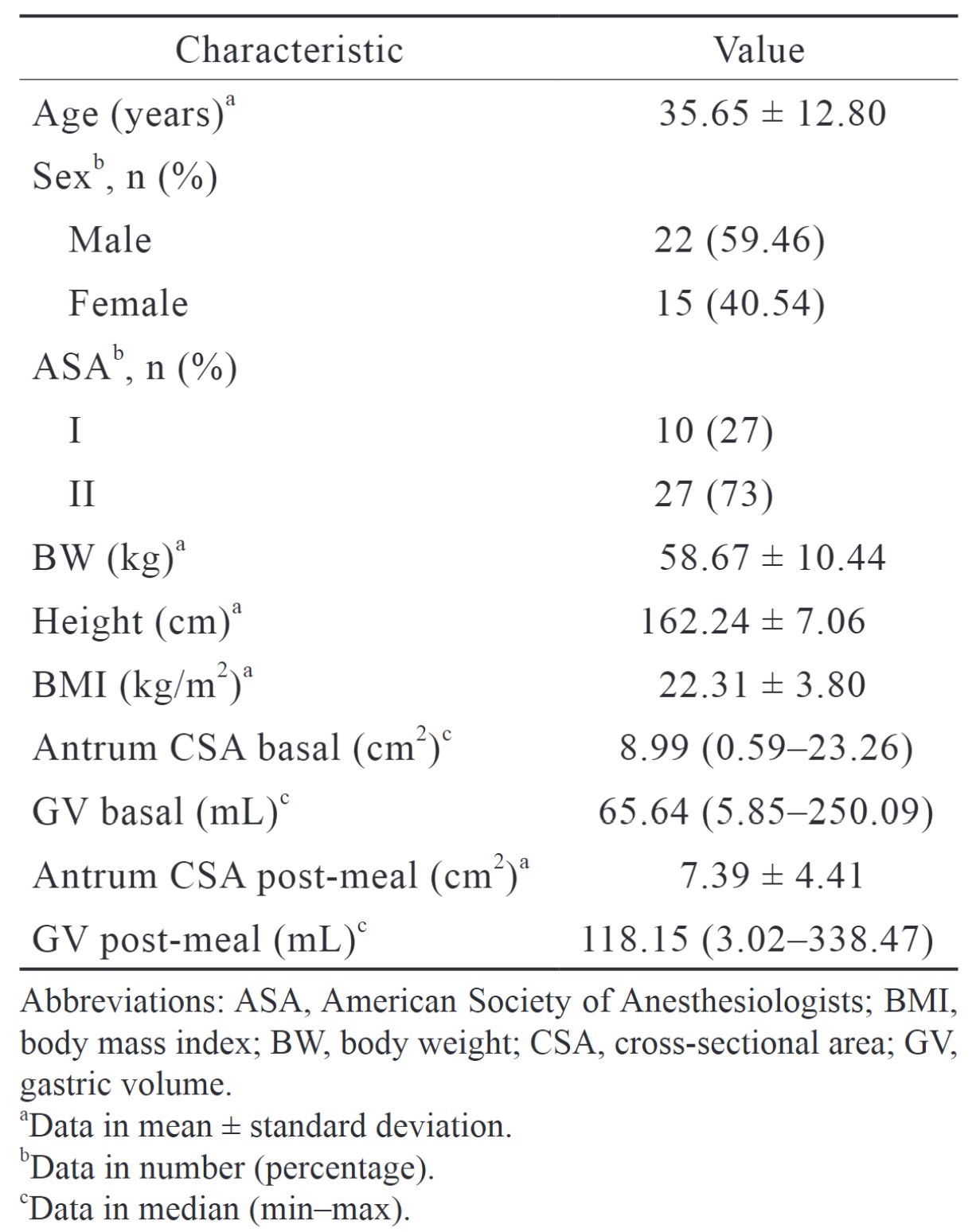
Of 68 patients who fit the inclusion criteria in this study, 31 were excluded, and 37 subjects were recruited for this study. All the subjects finished the dinner provided by the hospital. The subject characteristics of the subjects are presented in Table 2.
Download full-size image
From all the characteristics data, we did the bivariate analysis using Mann-Whitney for categorical variables (sex and ASA physical status) and Spearman for numerical variables (age, weight, height, and intima-media thickness [IMT]) to GV after 6 hours and 8 hours. The significant correlations were age and height for GV after fasting of 6 hours (
From all the variables that were significant in the bivariate analysis, we ran them for linear regression analysis. There were no significant variables in the GV for fasting of 6 hours. Still, the age variable was significant in the GV for the fasting of 8 hours (
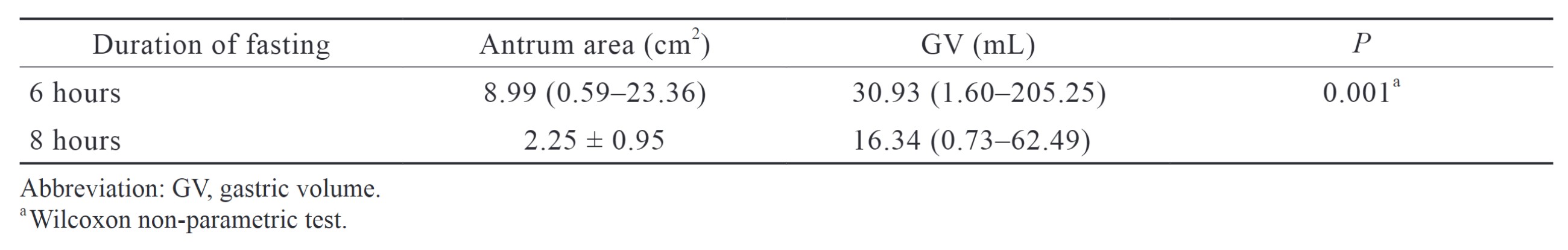
We found that subjects in the 8-hour fasting group had statistically lower GV than those in the 6-hour fasting (30.93 [1.60–205.25] mL vs. 16.34 [0.73–62.49 mL];
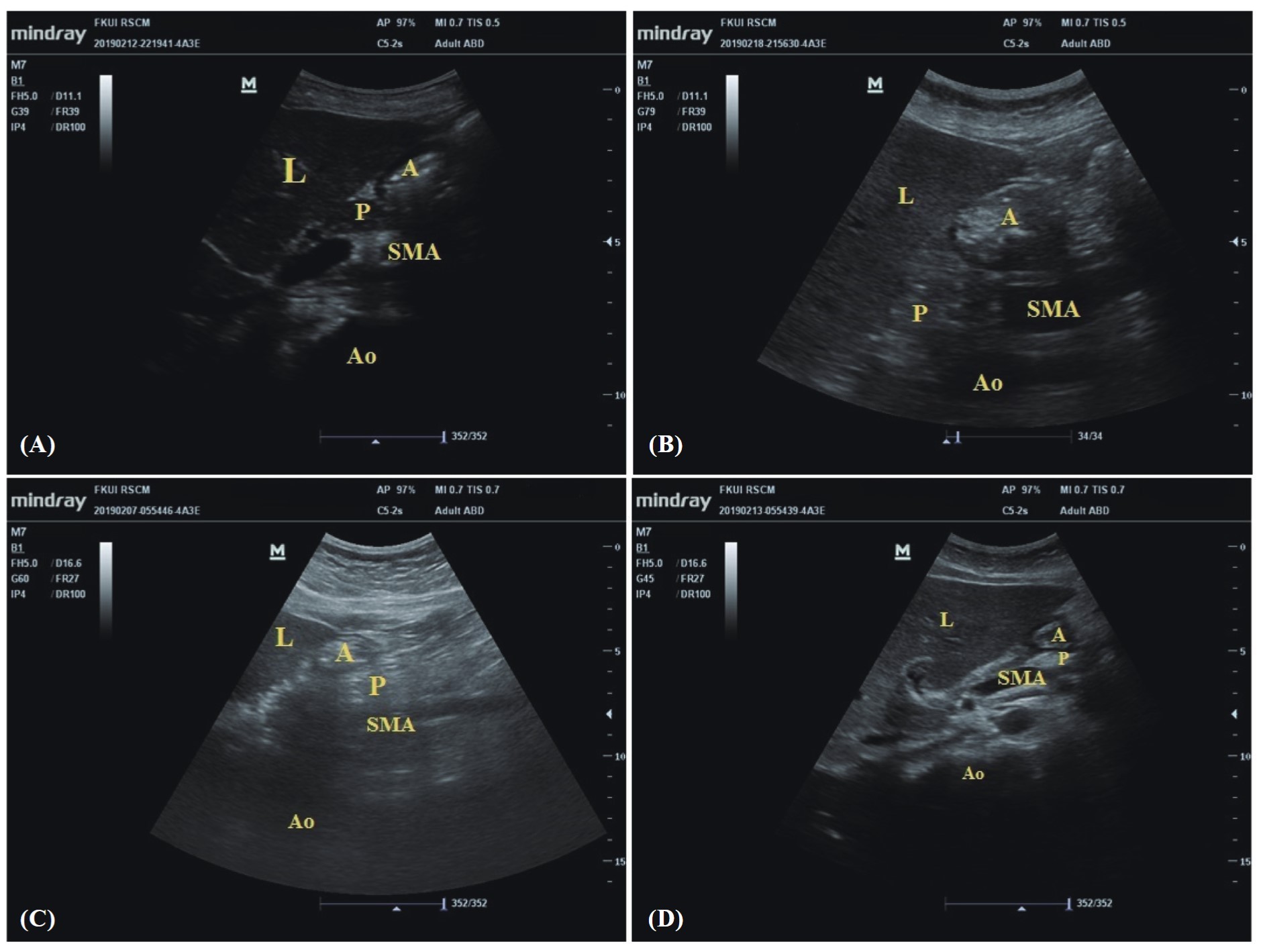
Download full-size image
(A) before fasting, (B) postprandial, (C) after fasting of 6 hours, (D) after fasting of 8 hours; (L) liver, (A) antrum, (P) pancreas, (SMA) superior mesenteric artery, (Ao) aorta.
Download full-size image
Discussion
Consideration for the timing of surgery and airway management is based on the last meal consumed. Six hours of fasting was considered sufficient for gastric emptying, specifically after a carbohydrate-based diet. Generalization could be problematic since, besides the food type, we also have to consider the patient’s comorbidities and other related conditions.2,3 In some instances, 8-hour fasting may not be sufficient in patients with impaired motility gastric or another gastrointestinal (GI) disease. In the case of an emergency, we also find it challenging to determine the ideal fasting time. Even in some cases, the stomach is still full even after the patient has fasted for an extended period.3 This condition requires an objective GV examination to identify the risk of aspiration and determine the airway management appropriate before anesthesia.3,5,6
Ultrasound of the stomach is one of the modalities used by gastroenterologists in the last 20 years to assess motility and gastric emptying and detect lesions (cancer) on the stomach wall.3 Ultrasound visualization of the gastric antrum can show us the stomach’s contents and help to differentiate between solid and liquid. Solid food in the stomach during anesthesia increases the risk of aspiration.6 Fluid GV can be calculated using existing mathematical models based on ultrasound measurement.
There were two formulas available for GV calculation; one was from Bouvet et al.7, and the other we used in this study from Perlas et al.4 The formula from Bouvet et al.7 can be used to calculate the GV under 250 mL in non-gravid adults with an IMT ranging from 14 to 31 kg/m2. The formula from Perlas et al.4 can only measure the GV up to 500 mL, but it can be used in adults aged 18–85 years old, with height over 145 cm, BW ranging from 40–110 kg, IMT < 35 kg/m2, and ASA status of I–III. The limitations of this formula are not available for patients with upper GI system anatomy abnormality, gastric ulcer, upper GI bleeding, vagal nerve denervation, diabetes, infiltrative diseases such as scleroderma or amyloidosis, medullar lesion above T10, or pregnancy.4 Gastroesophageal reflux, however, was not an exclusion.4
This study found that 94.6% of the patients had sufficient fasting after fasting of 6 hours. This number is close to 100% sufficient, as seen in fasting of 8 hours. Therefore, it is reasonable to say that 6-hour fasting also provides a safe time, especially for Indonesian or typical Asian food mainly consisting of rice and low-fat contents (25%). The perioperative aspiration incident is the outcome recorded during this study. The outcome of this study made all the subjects finish fasting of 8 hours, so with 100% sufficient fasting according to gastric ultrasound; there were no incidents of perioperative aspiration. Every incident of perioperative aspiration in our hospital was closely audited. Fasting is only one of many factors involved in this complication.
Interestingly, after calculation with Perlas et al.4 formula, the calculated GV resulted in negative values. These findings were seen in most subjects over 45 years old. The subtraction from the CSA value has proved too small to match the formula that depends on the constant number times the age in years. We analyzed the absolute value by omitting the negative mark on every calculated GV. Another criterion we can use to determine the risk of perioperative aspiration is the absolute CSA of less than 340 mm2, as used in the study by Kaydu and Gokcek3. When we used this criterion, the proportion of sufficient fasting in 6 hours was 56.8% and 89.2% in 8 hours (McNemar test,
Since the sample size for this study is small, we can analyze the two subjects to provide other factors that may contribute to the insufficient GV after fasting of 6 hours. Both of these subjects’ measurements were outliers with extreme values during the assessment of 6-hour fasting. One subject is a 38-year-old patient diagnosed with chronic osteoarthritis; the other is a 46-year-old diagnosed with urethral stricture and AIDS. HIV infection (human immunodeficiency virus) can slow gastric emptying, especially in patients with an advanced stage of GI infections.8 Visceral neuropathy in the early stages of HIV infection can also prolong gastric emptying.8 Another possible cause is gastroparesis, which can be idiopathic. It is characterized by impaired gastric emptying without apparent organic cause.9 The diagnosis of idiopathic gastroparesis, however, comes after any organic causes that the patient might suffer are excluded.
Age is one of the factors that can affect the antrum surface area and GV. In this study, age is a consistent factor that correlates either by bivariate analysis or multivariate analysis to the GV after fasting of 6 and 8 hours. This means that the older patients significantly had lower GV without any correlation to the Antral CSA in both periods of fasting. These findings differed slightly from what Perlas et al.4 stated that the antral CSA of elderly patients was greater than that of young patients, which resulted in lower calculated GV according to the formula. Kaydu and Gokcek3 also showed that the antral CSA after fasting of 8 hours was increased and had a low linear correlation with advancing age. Higher compliance of the gastric wall and prolonged gastric motility in older patients contributed to the higher GV than in younger patients.3,4 The different findings were because Perlas et al.4 measured the antral CSA during a full stomach setting after consuming various volumes of apple juice. Kaydu and Gokcek3 measured the antral CSA in the supine position. The gravitational fluid shift made the antral CSA larger in the right lateral than in the supine position. We chose the right lateral CSA because it is more sensitive than the supine position to detect low-volume states.4
The limitation of this study was that the primary gastric ultrasound examination was carried out only by one anesthesiologist who had prior training in gastric ultrasound. The second measurement was done by remeasuring the saved images. The mean values from both measurements were input as the final measurements. Since the images were not reacquired, there were not many differences in both measurements (ranging from 1 to 3 mL). Another study involved two independent radiologists to assess the antrum. The result difference between the two examiners ranged from 1 to 13 mL on an empty stomach and 2–85 mL after patients ate the standard meal.10
Ultrasound examination helps determine the risk of aspiration, especially in emergencies. However, gastric ultrasound examination requires special skills, so prior training is needed to ensure accurate examinations. Moreover, research subjects on gastric ultrasound examination have been validated only for non-gravid adults. Pediatric and obstetric patients still need further research to validate ultrasound examination of the stomach.
Acknowledgments
There is no conflict of interest in this study. This research is not sponsored or assisted by any party.
References
| 1 |
Van de Putte P, Perlas A.
Ultrasound assessment of gastric content and volume.
Br J Anaesth. 2014;113(1):12-22.
|
| 2 |
Apfelbaum JL, Agarkar M, Connis RT, Coté CJ, Nickinovich DG, Warner MA.
Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration.
Anesthesiology. 2017;126(3):376-393.
|
| 3 |
Kaydu A, Gokcek E.
Preoperative assessment of ultrasonographic measurement of antral area for gastric content.
Med Sci Monit. 2018;24:5542-5548.
|
| 4 |
Perlas A, Mitsakakis N, Liu L, et al.
Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination.
Anesth Analg. 2013;116(2):357-363.
|
| 5 |
Sharma S, Deo AS, Raman P.
Effectiveness of standard fasting guidelines as assessed by gastric ultrasound examination: a clinical audit.
Indian J Anaesth. 2018;62(10):747-752.
|
| 6 |
Perlas A, Davis L, Khan M, Mitsakakis N, Chan VWS.
Gastric sonography in the fasted surgical patient: a prospective descriptive study.
Anesth Analg. 2011;113(1):93-97.
|
| 7 |
Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D.
Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume.
Anesthesiology. 2011;114(5):1086-1092 doi:10.
|
| 8 |
Neild PJ, Nijran KS, Yazaki E, et al.
Delayed gastric emptying in human immunodeficiency virus infection: correlation with symptoms, autonomic function, and intestinal motility.
Dig Dis Sci. 2000;45(8):1491-1499.
|
| 9 |
Konturek JW, Fischer H, van der Voort IR, Domschke W.
Disturbed gastric motor activity in patients with human immunodeficiency virus infection.
Scand J Gastroenterol. 1997;32(3):221-225.
|
| 10 |
Ricci R, Bontempo I, Corazziari E, Bella AL, Torsoli A.
Real time ultrasonography of the gastric antrum.
Gut. 1993;34(2):173-176.
|