A remarkable proportion of patients with coronavirus disease 19 (COVID-19) develop severe respiratory failure and require mechanical ventilation, often achieving the criteria for severe acute respiratory distress syndrome (ARDS). They characteristically have diversified lung injury uniformly seen in patients with ARDS.1 Way back in 1974, Brayn2 recommended prone positioning (PP) in patients with hypoxic respiratory failure for a better outcome but improved survival was noted only after 40 years. PP in mechanically ventilated patients with ARDS is an evidence-based practice with the established fact of increased oxygenation.3 In confirmed moderate to severe ARDS, PP decreased the mortality by half (16.0% vs. 32.8%).3 Meta-analysis suggested that early PP for 12–16 hours/day combined with low tidal volume ventilation reduces mortality in severe hypoxic respiratory failure.4
Improved oxygenation observed with PP in typical ARDS is due to reduced ventilation-perfusion mismatching, hypoxemia, and shunting. Various mechanisms can be ascribed to these as follows: 5-7
(1) Generation of more homogenous lung aeration and strain distribution, thus enhancing recruitment of dorsal lung region due to reduction in the pleural pressure gradient between ventral and dorsal lung regions as a result of gravitational effects and geometrical shape matching of the lung to the chest cavity.
(2) Help to mitigate the effect of positive end-expiratory pressure which causes overdistension of previously well-ventilated alveoli, thereby reducing the ventilator-induced lung injury.
(3) Preservation of ventilatory homogeneity without alteration of regional distribution of blood flow thus leading to a reduction in shunting. Dorsal lung regions have a higher density of blood vessels (which is independent of gravity). Proning improves the ventilation in these nondependent lung fields, thereby improving the ventilation: perfusion (V:Q) matching, increased perfusion toward the anterior alveoli; improving the V:Q ratio.
(4) Change in regional diaphragmatic motion.
(5) Increase in functional residual capacity.
(6) Improved drainage of secretion.
Though PP is recommended by international guidelines for use in moderate to severe ARDS, observational data collected globally revealed that PP was employed in only 5.9% of mild, 10.3% of moderate, and 32.9% of severe ARDS. Hypoxia not severe enough to justify using PP or hemodynamic instability were the main reasons mentioned in the study for its restricted use.8 Despite being described in 1977, using PP in awake spontaneously breathing patients having ARDS was not popular in the era of pre-COVID-19.3 Valter et al.5 applied PP in 4 spontaneously breathing patients with hypoxemic respiratory failure in whom invasive mechanical ventilation (IMV) was indicated. He demonstrated a rapid increase in PaO2 while refraining from mechanical ventilation and its associated complications. All patients tolerated PP well.5 Recent studies also demonstrated avoidance of intubation with early and repeated application of PP with non-invasive ventilatory therapy, especially in patients with moderate ARDS.9,10
With the increasing load of COVID-19 cases, interest has grown in the potential for awake PP of patients to improve gas exchange and reduce the need for IMV and thus decrease the load on the intensive care unit (ICU). The physiological effects of awake PP in patients with acute respiratory failure are not well understood. However, studies suggested that PP can exert favorable physiological effects in awake healthy volunteers and volunteers under hypergravity conditions in the form of an increase in overall lung volume by 17% in PP compared to supine.11,12 PP was being associated with a reduction in regional transpulmonary pressure gradients and decreased regional ventilation/perfusion heterogeneity, largely because of more homogeneous ventilation owing to an increased functional residual capacity and a reduced gravitational gradient of ventilation in a volunteer study using functional lung imaging with proton magnetic resonance imaging to assess regional ventilation.10
The findings from proned healthy volunteers may not be universally applied to hypoxemic patients who are undergoing awake proning. Hypoxia leading to forceful inspiratory efforts with low tidal volume can generate patient self-inflicted lung injury in spontaneously breathing patients which is comparable to ventilator-induced lung injury. Non-invasive ventilation (NIV) combined with PP can attenuate this detrimental effect due to active participation of muscular diaphragm resulting in uniform distribution of stress as well as reduced regional lung stress due to decreased chest wall compliance.7,12
Presently evidence in the form of cohort studies or case series is available which depicted improved oxygenation after self-PP in COVID-19 cases.13-16 Consequently, globally many centers started practicing awake PP from the emergency room itself.14 Also, PP is applied by many in patients requiring O2 therapy as well as in ICU patients with a high-frequency nasal cannula (HFNC)/NIV.15-17 Early and frequent proning in COVID-19 patients has also been recommended by Anand et al.17 in a recently done systematic review of 13 studies and found a 30% reduction in the need for intubation.
Awake proning is also being included in guidelines for the management of COVID-19 pneumonia treatment protocols by Massachusetts General Hospital as well as also being advocated by the UK Intensive Care Society (ICS) as a standard of care for suspected or confirmed COVID-19 patients requiring FiO2 > 28%.18,19 It is now well justified that PP should be started as early as possible in patients with a diagnosis of COVID-19 pneumonia. If the patients are already habituated to proning, it becomes easy for them to be accustomed to the same once they require O2 therapy followed by HFNC/NIV. ICS guidelines promote cyclic proning every 30 minutes to 2 hours from proning to left lateral, 30–60° sitting up, right lateral to proning again.19 This cyclical change in position is usually well tolerated by the patients. Increased duration of proning may be achieved by incentive being given to the patient to attain target SpO2 while maintaining PP.
Though lacking formal evidence of outcome after PP in COVID-19 cases, it appears to be safe and well-tolerated. Constant encouragement of the patients and avoiding delay in intubation when the need arises are the key factors using PP as “rescue” in the management of patients with COVID-19 pneumonia. Changes in oxygenation shall be regularly monitored in non-intubated patients with PP, and the patient should be as closely monitored as an intubated patient would be. Care shall be taken to prevent pulling out of venous catheters or nasal cannula or obstruction of the nasal cannula while patients change the position. All patients shall be explained not to get proned immediately after having their meal to prevent gastroesophageal reflux. It must be avoided in patients with the need for immediate intubation, hemodynamic instability, unstable spine, or recent abdominal surgery and should be cautiously used in patients having morbid obesity, advanced pregnancy, seizures, facial injury, etc.
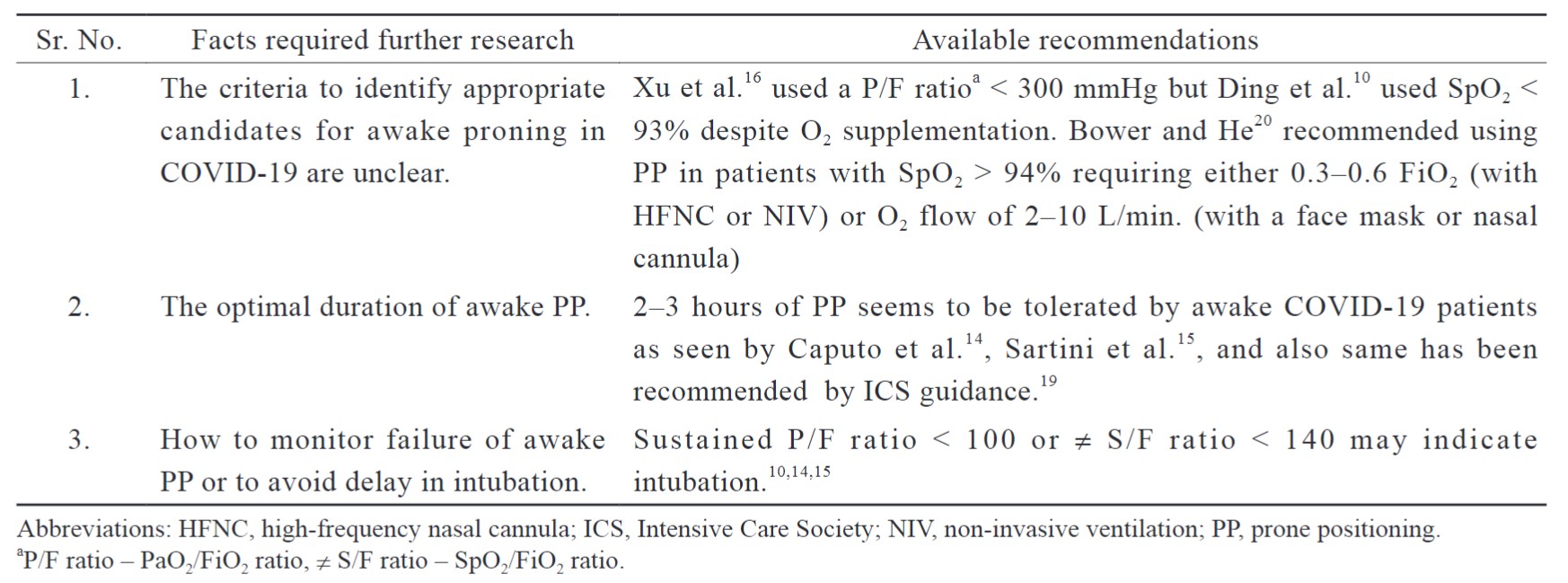
Still, more research is required to dig deep inside the topic (Table 1).
Download full-size image
I am looking forward to upcoming clinical trials to answer queries related to benefits, clinical outcomes, optimal frequency and duration, criteria for stopping, and hazards of awake PP in patients having COVID-19 pneumonia.
References
| 1 |
Ziehr DR, Alladina J, Petri CR, et al.
Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study.
Am J Respir Crit Care Med. 2020;201(12):1560-1564.
|
| 2 |
Brayn AC.
Comments of a devil’s advocate.
Am Rev Respir Dis. 1974;110(6P2):143-144.
|
| 3 |
Guérin C, Reignier J, Richard JC, et al.
Prone positioning in severe acute respiratory distress syndrome.
N Engl J Med. 2013;368(23):2159-2168.
|
| 4 |
Munshi L, Del Sorbo L, Adhikari NKJ, et al.
Prone position for acute respiratory distress syndrome.
Ann Am Thorac Soc. 2017;14(Supplement_4):S280-S288.
|
| 5 |
Valter C, Christensen AM, Tollund C, Schønemann NK.
Response to the prone position in spontaneously breathing patients with hypoxemic respiratory failure.
Acta Anaesthesiol Scand. 2003;47(4):416-418.
|
| 6 |
Gattinoni L, Taccone P, Carlesso E, Marini JJ.
Prone position in acute respiratory distress syndrome.
Am J Respir Crit Care Med. 2013;188(11):1286-1293.
|
| 7 |
Gattinoni L, Mascheroni D, Torresin A, et al.
Morphological response to positive end expiratory pressure in acute respiratory failure.
Intensive Care Med. 1986;12(3):137-142.
|
| 8 |
Guérin C, Beuret P, Constantin JM, et al.
A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study.
Intensive Care Med. 2018;44(1):22-37.
|
| 9 |
Scaravilli V, Grasselli G, Castagna L, et al.
Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study.
J Crit Care. 2015;30(6):1390-1394.
|
| 10 |
Ding L, Wang L, Ma W, He H.
Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study.
Crit Care. 2020;24(1):28.
|
| 11 |
Henderson AC, Sá RC, Theilmann RJ, Buxton RB, Prisk GK, Hopkins SR.
The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung.
J Appl Physiol (1985). 2023;115(3):313-324.
|
| 12 |
Rohdin M, Petersson J, Mure M, Glenny RW, Lindahl SGE, Linnarsson D.
Distributions of lung ventilation and perfusion in prone and supine humans exposed to hypergravity.
J Appl Physiol (1985). 2004;97(2):675-682.
|
| 13 |
Gattinoni L, Caironi P.
Prone positioning: beyond physiology.
Anesthesiology. 2010;113(6):1262-1264.
|
| 14 |
Caputo ND, Strayer RJ, Levitan R.
Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic.
Acad Emerg Med. 2020;27(5):375-378.
|
| 15 |
Sartini C, Tresoldi M, Scarpellini P, et al.
Respiratory parameters in patients with COVID-19 after using non-invasive ventilation in the prone position outside the intensive care unit.
JAMA. 2020;323(22):2338-2340.
|
| 16 |
Xu Q, Wang T, Qin X, Jie Y, Zha L, Lu W.
Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series.
Crit Care. 2020;24(1):250.
|
| 17 |
Anand S, Baishya M, Singh A, Khanna P.
Effect of awake prone positioning in COVID-19 patients—a systematic review.
Trends Anaesth Crit Care. 2021;36:17-22.
|
| 18 |
D’Souza FR, Murray JP, Tummala S, et al.
Implementation and assessment of a proning protocol for nonintubated patients with COVID-19.
J Healthc Qual. 2021;43(4):195-203.
|
| 19 |
Bamford P, Bentley A, Dean J, Whitmore D, Wilson-Baig N.
ICS guidance for prone positioning of the conscious COVID patient 2020.
https://emcrit.org/wp-content/uploads/2020/04/2020-04-12-Guidance-for-conscious-proning.pdf.. Accessed 31 December, 2020.
|
| 20 |
Bower G, He H.
Protocol for awake prone positioning in COVID-19 patients: to do it earlier, easier, and longer.
Crit Care. 2020;24(1):371.
|