Abstract
Background
The insufficient treatment of postoperative pain is considered a major barrier to enhanced patient recovery following surgery. Opioids remain the standard therapy for postoperative pain; however, the epidemic crisis of opioid abuse in the US has resulted in opioid-sparing multimodal analgesia (MMA) strategies in anesthesia practice. Complete perioperative pain management, particularly after discharge, may be undermined, resulting in chronic postsurgical pain. Thus, anesthesiologists and pain physicians should provide comprehensive MMA guidance for perioperative pain management.
Methods
The Taiwan Pain Society organized a working group, which included experts in the field of anesthesia, pain, and surgery. This group performed an extensive literature search, quality review, and drafted a consensus, which was discussed by experts and edited for feedback. Recommendations covered consent instruction, treatment interventions, intramuscular injection techniques, and prophylaxis for postoperative adverse events.
Results
This consensus included (1) a comparison of the pharmacology and pharmacokinetics between nalbuphine and dinalbuphine sebacate, (2) recommendations to help clinicians establish MMA with extended-release dinalbuphine sebacate injection, and (3) management of common adverse events during the perioperative pain period.
Conclusion
Extended-release dinalbuphine sebacate combined with the MMA strategy can reduce the medical burden and improve the quality of recovery following surgery.
Keywords
postoperative pain, consensus, nalbuphine, multimodal analgesia
Introduction
Approximately 70% of patients suffer from moderate to severe pain following surgery and the under-treatment of postoperative pain is considered a barrier to enhanced patient recovery and discharge from the hospital.1 Opioids represent an effective and safe way of managing pain when used appropriately; however, to address the opioid epidemic, the role of extended-release opioids in patients with acute pain following surgery is not well-characterized.2,3
Extended-release opioids have several advantages for treating acute pain, such as long-acting analgesia, relatively constant drug levels in the blood, less frequent analgesic administration required, and improved quality of recovery following surgery.4 However, before prescribing extended-release opioids to postsurgical patients, clinicians must evaluate their medical history, opioid-related side effects and overdose, drug-drug interactions, withdrawal, and risk of developing addiction to an individualized perioperative pain management plan.5
Currently, most extended-release opioids are full mu agonists, such as morphine, fentanyl, and oxycodone, which are associated with a higher risk of respiratory depression.6-8 The therapeutic component, nalbuphine, which is a kappa opioid receptor agonist and mu opioid receptor antagonist, is responsible for the long-duration analgesic effect of extended-release dinalbuphine sebacate (ERDS) injection. Based on its mechanism of action, combining low concentrations of nalbuphine with full mu agonists not only reduces the overall analgesic effects, but also decreases the incidence of adverse reactions associated with full mu agonists, such as pruritus.9
The purpose of the collaborative document from this working group, based on a literature review and expert opinion, is to serve as an educational resource for anesthesiologists and pain physicians that are focused on multimodal analgesia (MMA) with ERDS during the perioperative period. This study provides information on the pharmacology of nalbuphine and ERDS, an overview of the ERDS literature, and evidence-based recommendations. It is not intended to serve as a comprehensive guide for MMA or postoperative pain management, but suggests a role for ERDS in postoperative MMA.
Methods
Committee Composition
The Taiwan Pain Society (TPS) convened a working group consisting of experts in anesthesia and/or pain medicine and surgery to review evidence and formulate recommendations on MMA with ERDS in perioperative pain management. Two co-chairs (W.Z. Sun and C.S Wong) were selected to lead the working group, organize a discussion, and draft a consensus.
Target Audience and Scope
The goal of the consensus was to provide evidence-based recommendations for MMA with ERDS during the perioperative period. The target audience is all clinicians, including anesthesiologists and pain physicians, who plan to integrate ERDS into MMA for perioperative pain management. The management of chronic and nonsurgical pain are outside the scope of this article.
Evidence Search and Review
The consensus was informed by evidence review and expert opinion. The working group developed inclusion criteria to guide the evidence review process. A literature search was performed through PubMed with the chemical and brand name of ERDS on May 2023. Eleven articles were included in the evidence review, including a case report, review, and randomized studies. There was no limitation to the type of articles, but the quality of each source was weighed by the working group.
Grading of the Evidence and Recommendations
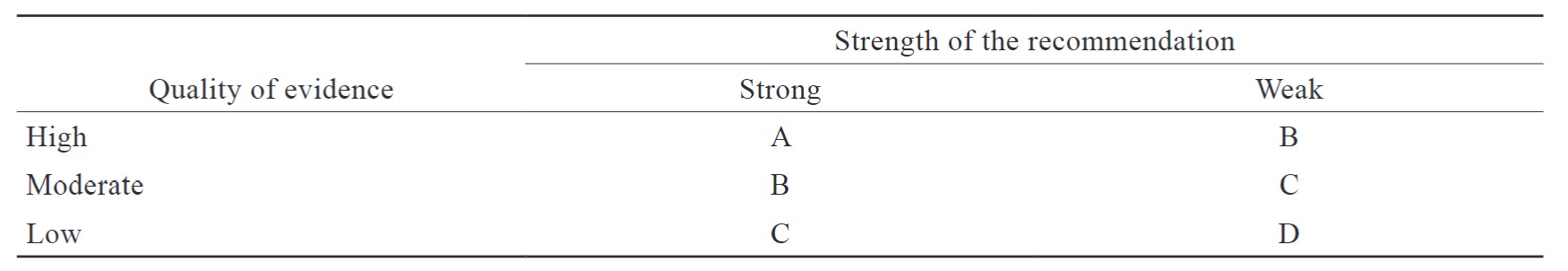
The group established methods to rate the recommendations in this consensus. Each recommendation was rated by its strength (strong and weak) and evidence quality (high, moderate, low).
Consensus Development Process
Using the available evidence, the working group developed a draft document for MMA with ERDS, including a treatment regimen, timing of administration, and adverse event management. The document was then sent to TPS and edited based on feedback from the individual members.
Results
Pharmacology
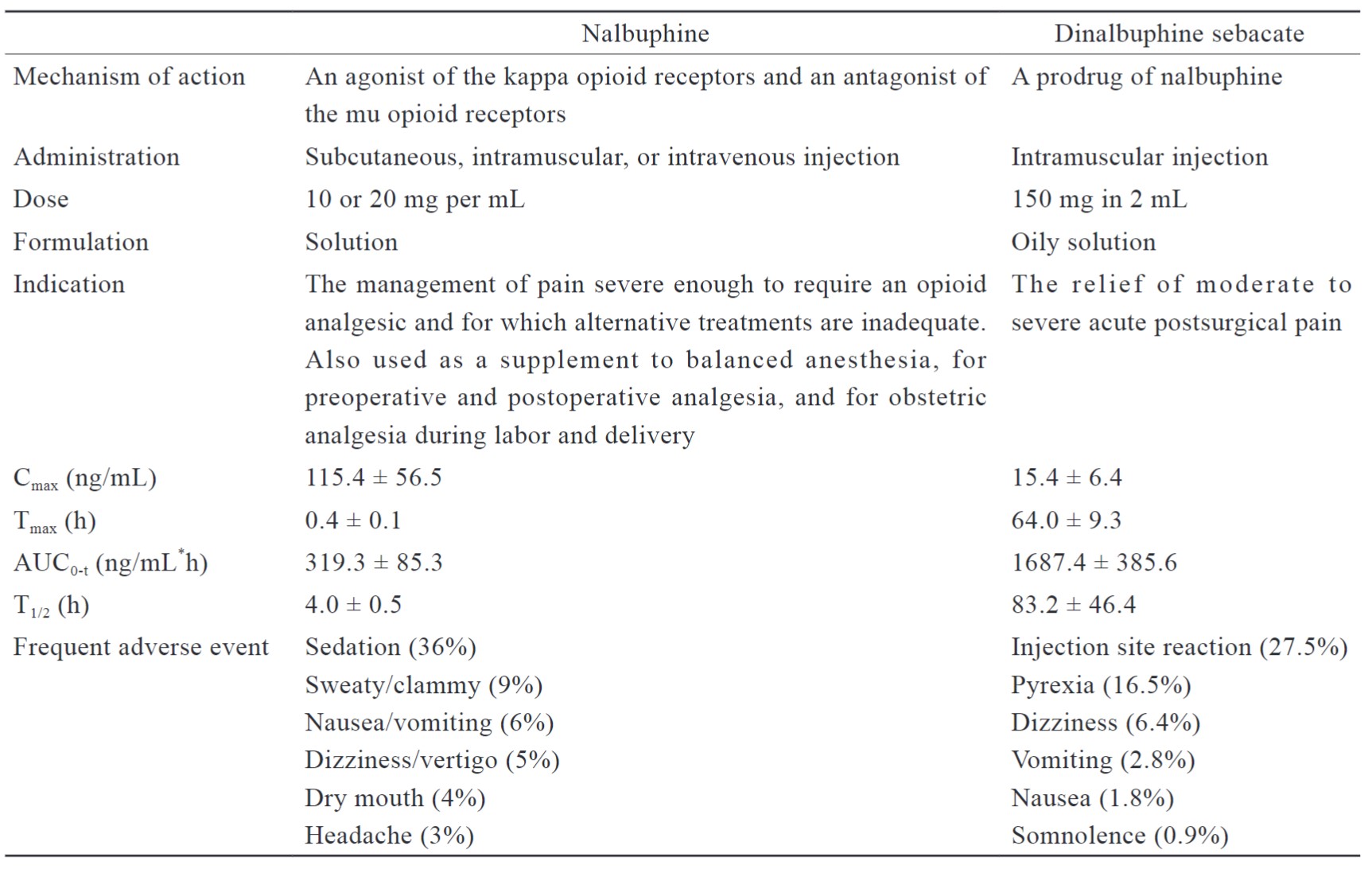
Nalbuphine is a short-acting analgesic used for postoperative pain management, as anesthesia for preoperative and postoperative analgesia, and a supplement for analgesia during labor and delivery.10 ERDS is a long-acting nalbuphine prodrug with a mixed opioid agonist and an antagonist that offers a lower risk of abuse or withdrawal, and provides a ceiling effect on spontaneous respiratory depression.11 When used to relieve postoperative pain, ERDS may be administered as a single intramuscular injection without dose adjustment. There are no restrictions on its concomitant use with other opioid anesthetics or analgesics, and it has the advantage of merging into MMA. Thus, utilizing drugs with multiple mechanisms of action to target pain pathways to successfully alleviate pain. The pharmacology and pharmacokinetic characteristics of nalbuphine and dinalbuphine sebacate are summarized in Table 1.
Download full-size image
Confusion is often associated with the pharmacological properties of ERDS, most notably with respect to antagonism with other opioid medications. The pharmacological profile of ERDS includes reduced intrinsic activity toward mu receptors, resulting in a lower potential of antagonism, even when ERDS are administered prior to surgery.12 Based on previous studies combining nalbuphine with opioids, low plasma levels of nalbuphine not only provide adequate postoperative analgesia, but also reduce the risk of opioid-induced adverse events (OIAEs), such as pruritus.13,14
Literature Review
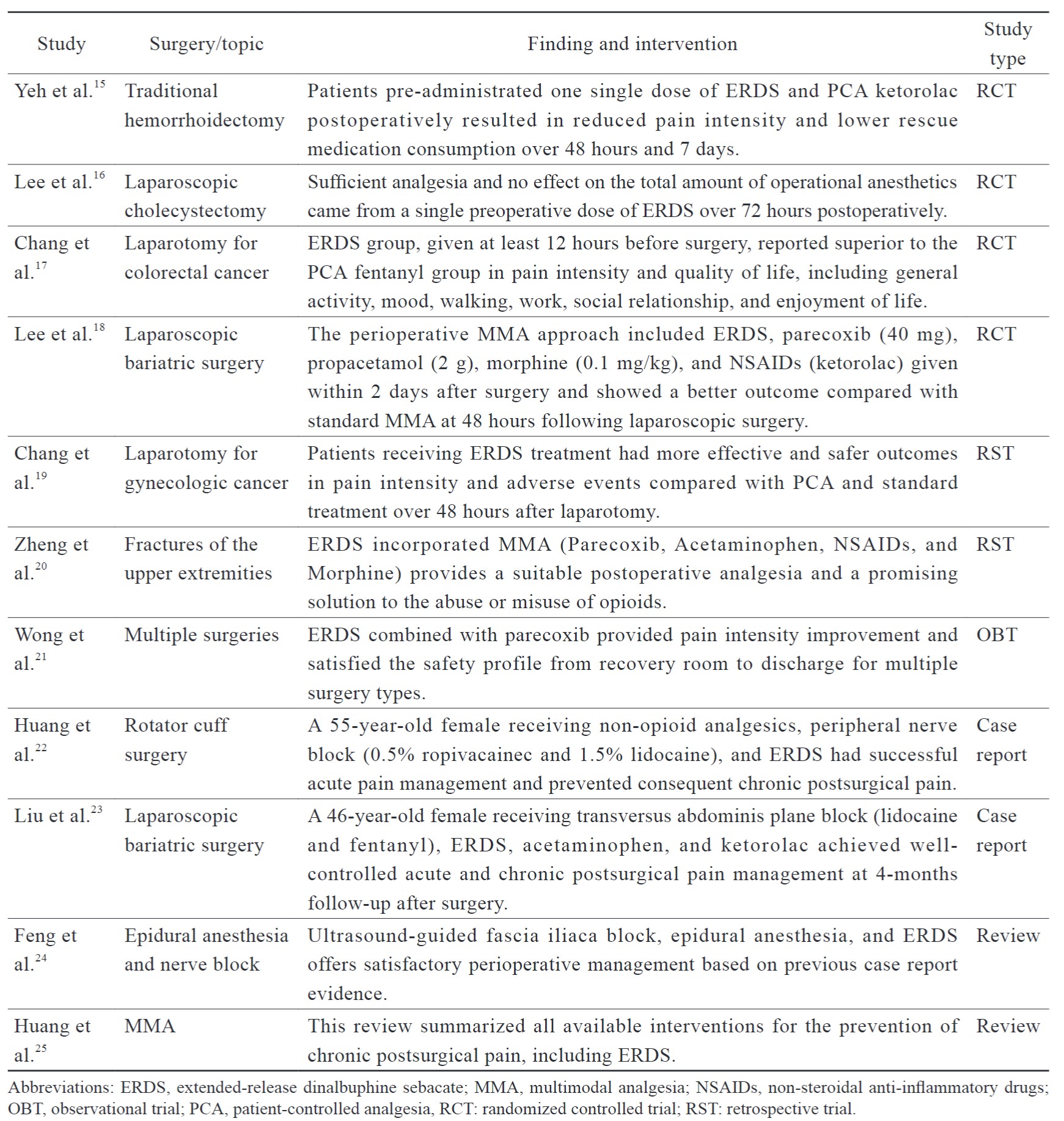
A literature search was performed based on pre-defined criteria, and all of the selected articles were qualified by their evidence and strength of recommendation (Table 2). Table 3 summarizes the findings of perioperative management of patients on ERDS for postoperative pain. These studies consisted of reviews, case reports, retrospective studies, and randomized controlled trials. Most studies administrated ERDS one day before surgery, whereas a few were given on the day of surgery. The ERDS-integrated MMA approach demonstrated benefits of efficacy and safety for several laparotomy and laparoscopic surgeries, including less pain intensity and rescue medication consumption. Chang et al.17 reported that ERDS improved postoperative pain intensity and quality of recovery compared with the patient-controlled analgesia (PCA) group in patients receiving colorectal laparotomy with less inference in the ward and more mobility, which enhanced early patient recovery. Similarly, a significant reduction in pain intensity using analgesics with multiple mechanisms was also effective for laparotomy in gynecologic cancer patients.19 Pre-administration ERDS did not affect the total amount of anesthetics consumed during operation, and severe opioid antagonism was not observed, such as sedation or respiratory depression. Zheng et al.20 demonstrated that ERDS incorporated into MMA offered a satisfactory solution to orthopedic operations in the upper extremity and also markedly decreased the possibility of misuse and abuse of opioids during the perioperative period.
Inadequate analgesia during the first week after laparoscopic surgery is recognized as a major risk factor for developing chronic postsurgical pain (CPSP). Lee et al.18 demonstrated that ERDS integrated with standard MMA has the potential to circumvent drug-related systemic complications and CPSP.
In addition, no evidence was found that preoperative administration of ERDS caused opioid antagonism or anesthesia dose increases during surgeries involving general anesthesia. With five-year post-marketing pharmacovigilance monitoring, there was no sedation or respiratory depression reported from spontaneous reporting or clinical trials.
Download full-size image
Download full-size image
Discussion
Recommendations
Preoperative Consent Instruction (Strong Recommendation, Low-Quality Evidence)
Clinicians should provide patient-centered, individually tailored instructions, including information on treatment options for managing postoperative pain, and the frequency and severity of postoperative adverse events. Preoperative consent and instructions were beneficial to patients with intensive needs (e.g., psychological comorbidities or financial issues), including less postoperative analgesic consumption and potential adverse events. Despite insufficient evidence to demonstrate the beneficial effects of preoperative instructions, experts believe such face-to-face instructions are valuable for helping patients to understand perioperative treatment.
To establish a perioperative pain management strategy, clinicians should conduct a preoperative evaluation that includes an assessment of medical and psychiatric comorbidities, concomitant medications, substance abuse, and previous postoperative treatment regimens and responses. The perioperative pain management plan should also consider current examinations and the surgical site. Whether the patient was enrolled in a clinical study or not, developing a well-individualized perioperative pain management plan is important.
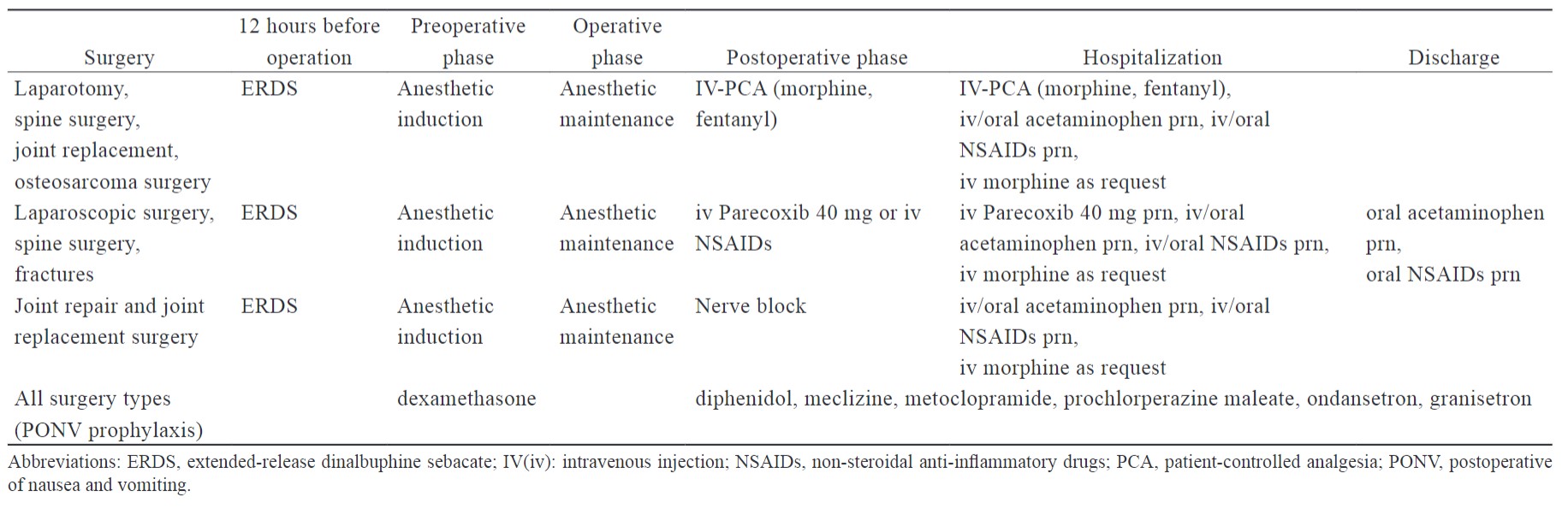
Clinicians may offer individual MMA with ERDS for the treatment of postoperative pain depending on different surgery types or patient situations. The interventions and administration time of ERDS-integrated MMA are summarized by surgery type in Table 4. ERDS served as the background analgesia in the MMA strategy, providing low levels of nalbuphine postoperatively on days five to seven. It can decrease the time of analgesic exposure needed and improve the quality of recovery with less interference by the patient.
Download full-size image
Three major ERDS-integrated MMA approaches include: (1) combination of opioids with PCA with morphine or fentanyl; (2) non-steroidal anti-inflammatory analgesics, such as parecoxib and ketorolac; and (3) peripheral nerve block during the postoperative phase.
ERDS exhibits a relatively slow release pharmacokinetic profile and requires at least 12 hours to reach the minimal analgesia plasma concentration. The timing of two administrations is based on the surgery type and day of hospitalization: 12 hours before operation or after anesthetic induction is completed. Based on pharmacokinetics, the ideal administration time of ERDS is at least 12 hours before the scheduled operation (Figure 1).26 Although surgery and hospitalization one day before operation may not be feasible, ERDS administrated between anesthetic induction and anesthetic maintenance represents an alternative option (Figure 2).
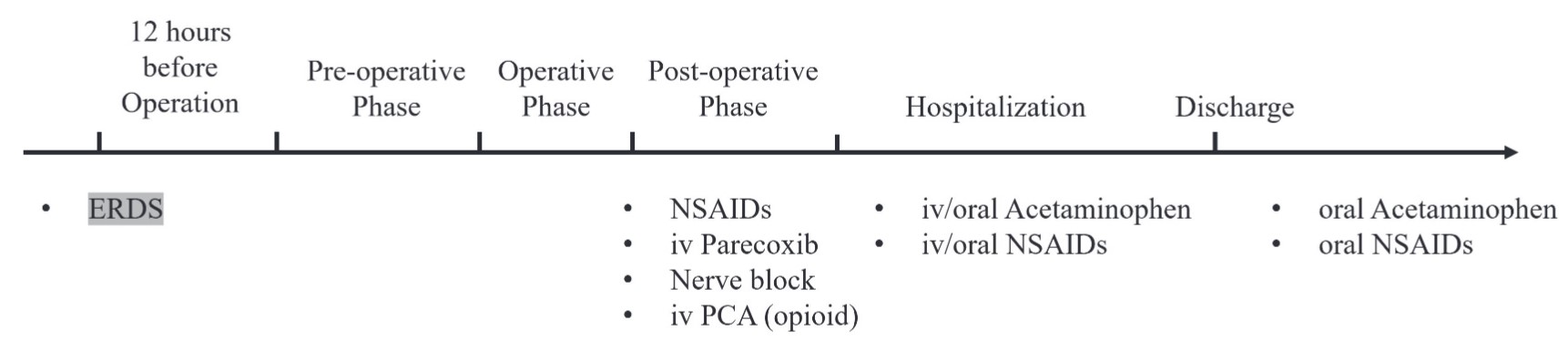
Download full-size image
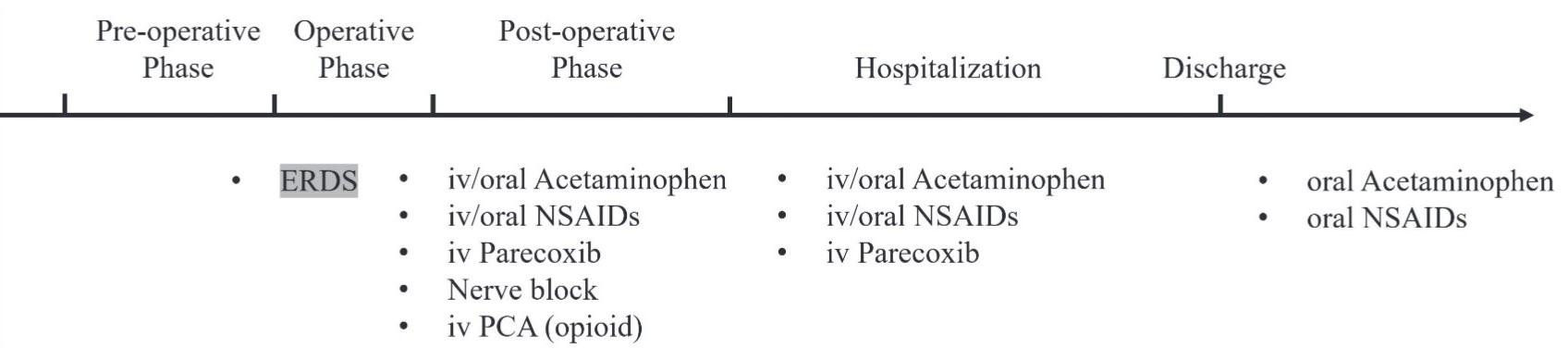
Download full-size image
Injection site reactions were common adverse events following intramuscular injection, especially for oily solutions. It is important to receive intramuscular injection training for clinicians and medical practitioners before they administeri ERDS. The injection site reaction can be minimized through ultrasound-guided injection, Z-track injection, and the appropriate needle length.27,28 According to five-year post-marketing pharmacovigilance, both the reporting rate of injection site reactions in spontaneous reports and the percentage of intramuscular injection-related reactions in post-marketing studies were significantly decreased after improving intramuscular injection training and the updated packaging, particularly for ultrasound-guided injections (Table 5).29
Clinicians should consider appropriate prophylaxis of postoperative nausea and vomiting (PONV). Patients with PONV may experience poor quality recovery, including more frequent antiemetic administrations for treatment as well as severe consequences and complications. The intervention for common postoperative adverse events is summarized in Table 5. Clinicians should also assess OIAEs, such as dizziness, pruritus, and constipation. The risk of OIAE was increased for patients who received systemic opioids for postoperative analgesia. With respect to sedation and respiratory depression, there were no similar adverse events reported from spontaneous or post-marketing studies involving more than 80,000 cases of exposure.29 Furthermore, limited evidence suggests that postoperative hydration may be useful for preventing PONV.30
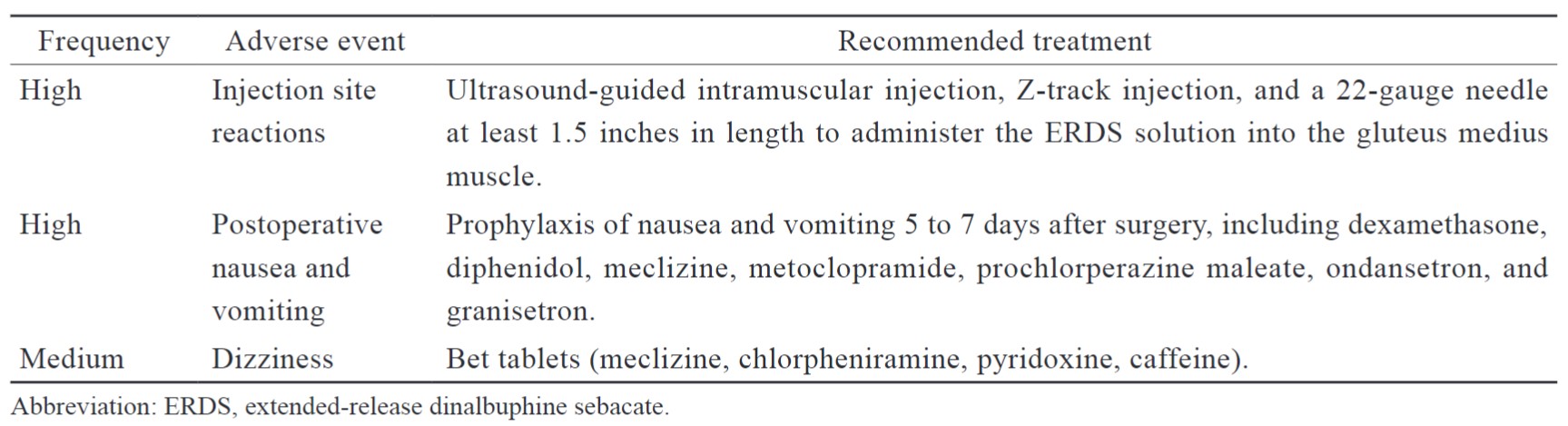
Download full-size image
Conclusion
After a review of the clinical evidence, the working group developed recommendations to improve MMA with ERDS in perioperative management. The recommendations were subsequently discussed and approved by TPS experts. This consensus provides training material for clinicians who would like to use ERDS in MMA and provide treatment interventions for general surgery and administration timing.
The most commonly reported adverse events occurring in MMA with ERDS were identified, and monitoring opioid-related adverse events and complications is necessary when patients are moved to the recovery room and ward. In addition, although the working group reviewed clinical evidence to support the benefit of ERDS in MMA, the specific components were dependent on the patient, setting, and surgery. Thus, the recommendations may not apply to all patients and situations. To optimize potential treatment options, there is also an urgent need to conduct research on practice gaps between evidence-based interventions and ideal perioperative management.
Acknowledgments
The authors would like to thank the TPS members who reviewed and corrected the recommendations of this consensus.
References
| 1 |
Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL.
Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey.
Curr Med Res Opin. 2014;30(1):149-160.
|
| 2 |
Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL.
Prescription opioid analgesics commonly unused after surgery: a systematic review.
JAMA Surg. 2017;152(11):1066-1071.
|
| 3 |
Kaafarani HMA, Han K, El Moheb M, et al.
Opioids after surgery in the united states versus the rest of the world: The International Patterns of Opioid Prescribing (iPOP) multicenter study.
Ann Surg. 2020;272(6):879-886.
|
| 4 |
Sloan P.
Update on extended-release opioids in pain management.
Expert Opin Drug Deliv. 2014;11(2):155-158.
|
| 5 |
Secrest MH, Phillips S, Cepeda MS, Kern DM, Esposito DB, Wedin GP.
Impact of the extended-release/long-acting opioid analgesics risk evaluation and mitigation strategy on prescribing practices.
J Opioid Manag. 2023;19(2):99-110.
|
| 6 |
Zeng Z, Lu J, Shu C, et al.
A comparision of nalbuphine with morphine for analgesic effects and safety: meta-analysis of randomized controlled trials.
Sci Rep. 2015;5:10927.
|
| 7 |
Algera MH, Kamp J, van der Schrier R, et al.
Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal.
Br J Anaesth. 2019;122(6):e168-e179.
|
| 8 |
Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A.
Non-analgesic effects of opioids: opioid-induced respiratory depression.
Curr Pharm Des. 2012;18(37):5994-6004.
|
| 9 |
Yeh YC, Lin TF, Lin FS, Wang YP, Lin CJ, Sun WZ.
Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain.
Br J Anaesth. 2008;101(4):542-548.
|
| 10 |
Par Pharmaceutical.
NUBAIN® (Nalbuphine hydrochloride) injection, for intramuscular, subcutaneous, or intravenous use.
|
| 11 |
Lumosa Therapeutics Co., Ltd.
NALDEBAIN® (Dinalbuphine Sebacate) ER Injection.
|
| 12 |
Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB.
The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine.
Life Sci. 1993;52(4):389-396.
|
| 13 |
Yeh YC, Lin TF, Chang HC, et al.
Combination of low-dose nalbuphine and morphine in patient-controlled analgesia decreases incidence of opioid-related side effects.
J Formos Med Assoc. 2009;108(7):548-553.
|
| 14 |
Jannuzzi RG.
Nalbuphine for treatment of opioid-induced pruritus: a systematic review of literature.
Clin J Pain. 2016;32(1):87-93.
|
| 15 |
Yeh CY, Jao SW, Chen JS, et al.
Sebacoyl dinalbuphine ester extended-release injection for long-acting analgesia: a multicenter, randomized, double-blind, and placebo-controlled study in hemorrhoidectomy patients.
Clin J Pain. 2017;33(5):429-434.
|
| 16 |
Lee SO, Huang LP, Wong CS.
Preoperative administration of extended-release dinalbuphine sebacate compares with morphine for post-laparoscopic cholecystectomy pain management: a randomized study.
J Pain Res. 2020;13:2247-2253.
|
| 17 |
Chang TK, Huang CW, Su WC, et al.
Extended-release dinalbuphine sebacate versus intravenous patient-controlled analgesia with fentanyl for postoperative moderate-to-severe pain: a randomized controlled trial.
Pain Ther. 2020;9(2):671-681.
|
| 18 |
Lee YE, Wang SY, Chen JH, et al.
Efficacy and safety of parenteral injection of an extended release κ-receptor opioid sebacoyl dinalbuphine ester for acute and chronic pain after laparoscopic bariatric surgery: a randomized, placebo-controlled, double-blind trial.
Obes Surg. 2023;33(4):1192-1201.
|
| 19 |
Chang SH, Chang TC, Chen MY, Chen WC, Chou HH.
Comparison of the efficacy and safety of dinalbuphine sebacate, patient-controlled analgesia, and conventional analgesia after laparotomy for gynecologic cancers: a retrospective study.
J Pain Res. 2021;14:1763-1771.
|
| 20 |
Zheng ZH, Yeh TT, Yeh CC, et al.
Multimodal analgesia with extended-release dinalbuphine sebacate for perioperative pain management in upper extremity trauma surgery: a retrospective comparative study.
Pain Ther. 2022;11(2):643-653.
|
| 21 |
Wong JON, Tan TDM, Chao MT, Yeh CH.
Nalbuphine sebacate combined with parecoxib was effective in the treatment of postsurgical pain: a preliminary observational study.
Taiwan J Pain. 2020;30(1):19-26.
|
| 22 |
Huang WH, Huang NC, Lin JA, Wong CS.
Multimodal analgesia for shoulder rotator cuff surgery pain: the role of Naldebain® and ultrasound-guided peripheral nerve blocks combination.
J Med Sci. 2020;40(6):279-283.
|
| 23 |
Liu SY, Ho YH, Wong CS.
Multimodal analgesia with long-acting dinalbuphine sebacate plus transversus abdominis plane block for perioperative pain management in bariatric surgery: a case report.
Front Pharmacol. 2021;12:683782.
|
| 24 |
Feng YP, Huang NC, Chang HJ, Wong CS.
The role of epidural anesthesia plus ultrasound-guided peripheral nerve block and naldebain in chronic postsurgical pain.
Taiwan J Pain. 2018;28(1):22-29.
|
| 25 |
Huang CC, Sun WZ, Wong CS.
Prevention of chronic postsurgical pain: the effect of preventive and multimodal analgesia.
Asian J Anesthesiol. 2018;56(3):74-82.
|
| 26 |
Tien YE, Huang WC, Kuo HY, et al.
Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation.
Biopharm Drug Dispos. 2017;38(8):494-497.
|
| 27 |
Yasuhara Y, Hirai E, Sakamaki S, et al.
Using ultrasonography in evaluating the intramuscular injection techniques used for administering drug treatments to schizophrenic patients in Japan.
J Med Invest. 2012;59(1-2):213-219.
|
| 28 |
Ogston-Tuck S.
Intramuscular injection technique: an evidence-based approach.
Nurs Stand. 2014;29(4):52-59.
|
| 29 |
Man KM, Lee SO, Lu CH, Wong CS, Sun WZ.
Injection site reactions before and after intramuscular injection technique revision: a postmarketing analysis of NALDEBAIN® from 2017 to 2022.
Asian J Anesthesiol. 2023;61(1):14-20.
|
| 30 |
Ismail EA, Bakri MH, Abd-Elshafy SK.
Dexamethasone alone versus in combination with intra-operative super-hydration for postoperative nausea and vomiting prophylaxis in female patients undergoing laparoscopic cholecystectomy: a randomized clinical trial.
Korean J Anesthesiol. 2017;70(5):535-541.
|