Abstract
Background
Inadequate postoperative analgesia may cause postoperative complications, such as pulmonary complications. This study evaluated the analgesic effectiveness of a single preoperative injection of dinalbuphine sebacate (DS) in patients undergoing video-assisted thoracoscopic wedge resection and assessed whether it can reduce the incidence of postoperative pulmonary complications (PPCs).
Methods
In this study, the data of 757 patients who underwent VATS wedge resection at a medical center were retrospectively reviewed. The patients were divided into the DS group and the conventional analgesia (CA) group. The following parameters were analyzed: analgesic consumption during hospitalization, the incidence of PPCs, and the postoperative use of oxygen therapy.
Results
Compared with the CA group, the DS group had lower nalbuphine, tramadol, parecoxib, acetaminophen, diclofenac, and utraphen consumption during the postoperative period; higher morphine and ketorolac consumption; and comparable fentanyl consumption. Nonetheless, the frequency of requesting pain relief was significantly lower in the DS group. No significant between-group differences were noted in the incidence of PPCs. However, the DS group had fewer requirements for oxygen therapy in the ward, early removal of chest tubes, and shorter length of hospital stay.
Conclusion
A single preoperative injection of DS reduced the frequency of salvage analgesic administration and total consumption of certain postoperative analgesics, suggesting the effective pain relief of DS, and it did not increase the incidence of PPCs. Additionally, it reduced the need for postoperative oxygen therapy, which may suggest a better prognosis and smoother postoperative pulmonary recovery for patients.
Keywords
dinalbuphine sebacate, postoperative analgesia, postoperative pulmonary complications, video-assisted thoracoscopic wedge resection
Introduction
Postoperative pulmonary complications (PPCs) are significant causes of morbidity and mortality after anesthesia and surgery.1 Risk factors for PPCs include patient comorbidities, age, sex, type of anesthesia, and type of surgery as well as thoracic surgery itself.2 Video-assisted thoracoscopic surgery (VATS) has become increasingly common, and it is associated with a lower incidence of PPCs than traditional thoracotomy.3-6 However, depending on how PPCs are defined, their incidence in VATS can range from 7.4% to 11.3%.7-9 PPCs are associated with prolonged hospital stay and high mortality.7,10
Uncontrolled postoperative pain produces a neuroendocrine stress response, resulting in elevated plasma catecholamine levels and subsequently leading to PPCs.11,12 Effective analgesia can reduce PPC incidence following thoracotomy, and thoracic epidural analgesia (TEA) has thus become the gold standard for postoperative pain management after thoracotomy.13,14 A higher averaged nociceptive response index during VATS surgery is associated with an increased rate of PPCs.15 Therefore, an appropriate analgesic plan may reduce PPCs after VATS.
Few recommendations have been made regarding pain management in VATS. The European Society of Regional Anesthesia & Pain Therapy16 has not recommended TEA as the gold standard for analgesia in VATS because alternative methods such as thoracic paravertebral block and intravenous (IV) patient-controlled analgesia have shown analgesic efficacy comparable to that of TEA, and they are less invasive, and no significant difference in adverse drug events has been observed.
Whether these methods of pain management influence PPCs incidence remains unknown. A study reported that in VATS procedures, both lack of fentanyl and excessive fentanyl administration during surgery were associated with an increased incidence of PPCs.17 Furthermore, some specific analgesic agents, such as gabapentinoids, or analgesic techniques that are effective in relieving postoperative pain may be associated with an increased risk of PPCs.18,19 Preoperative administration of gabapentinoids significantly increased the incidence of PPCs after total knee or hip replacement surgery20 and elective gynecologic surgery.21 Therefore, an analgesic plan for relieving postoperative pain sufficiently, which is not excessive or inadequate, without the development of PPCs in VATS is important.
Dinalbuphine sebacate (DS, Naldebain) is a new extended-release form of nalbuphine, which is a κ agonist/partial μ antagonist. After intramuscular injection, it is slowly released in the body and maintains a stable plasma concentration for up to 6 days, providing long-lasting pain relief without severe central respiratory depression.22 Compared with TEA, intramuscular injection is convenient, less technically demanding, and avoids the complications related to neuraxial analgesia or nerve block. It can also provide a more stable and nonfluctuating plasma concentration of effective analgesics than that obtained with conventional IV opioid analgesia, thus avoiding complications resulting from overly high peak plasma concentration, such as respiratory depression.
At our institution, DS is often used as the main component of multimodal analgesia (MMA) in patients undergoing VATS. In this study, we analyzed whether the preoperative administration of DS as the main component of MMA in patients undergoing VATS wedge resection can effectively achieve postoperative pain relief and avoid PPCs.
Methods
Patients and Ethics Approval
This is a retrospective cohort study. From the Asus Lumos database, we retrospectively obtained the data of hospitalized patients aged 20–65 who underwent VATS wedge resection at Chung Shan Medical University Hospital from January 1, 2020, to October 31, 2022. This study was approved by the Human Research Ethics Committee of Chung Shan Medical University Hospital (approval number: CS1-23005, the date of approval: September 23, 2023), and the requirement for patient consent was waived by the institutional ethics committee. This study was conducted in accordance with the principles of the Declaration of Helsinki.
We excluded patients (n = 157) with body mass index (BMI) ≥ 27, obstructive sleep apnea, or respiratory infections within 1 month before surgery as well as those requiring emergency surgery, as they are considered a high-risk group for PPCs. Finally, 757 patients were included in this study, of whom 618 received IV conventional analgesia (CA; the CA group) and 139 received preoperative DS (the DS group) as part of MMA. We replaced American Society of Anesthesiologists (ASA) classification with Charlson Comorbidity Index (CCI) because the database lacked ASA classification for patients. Figure 1 provides a summary of the patient selection process.
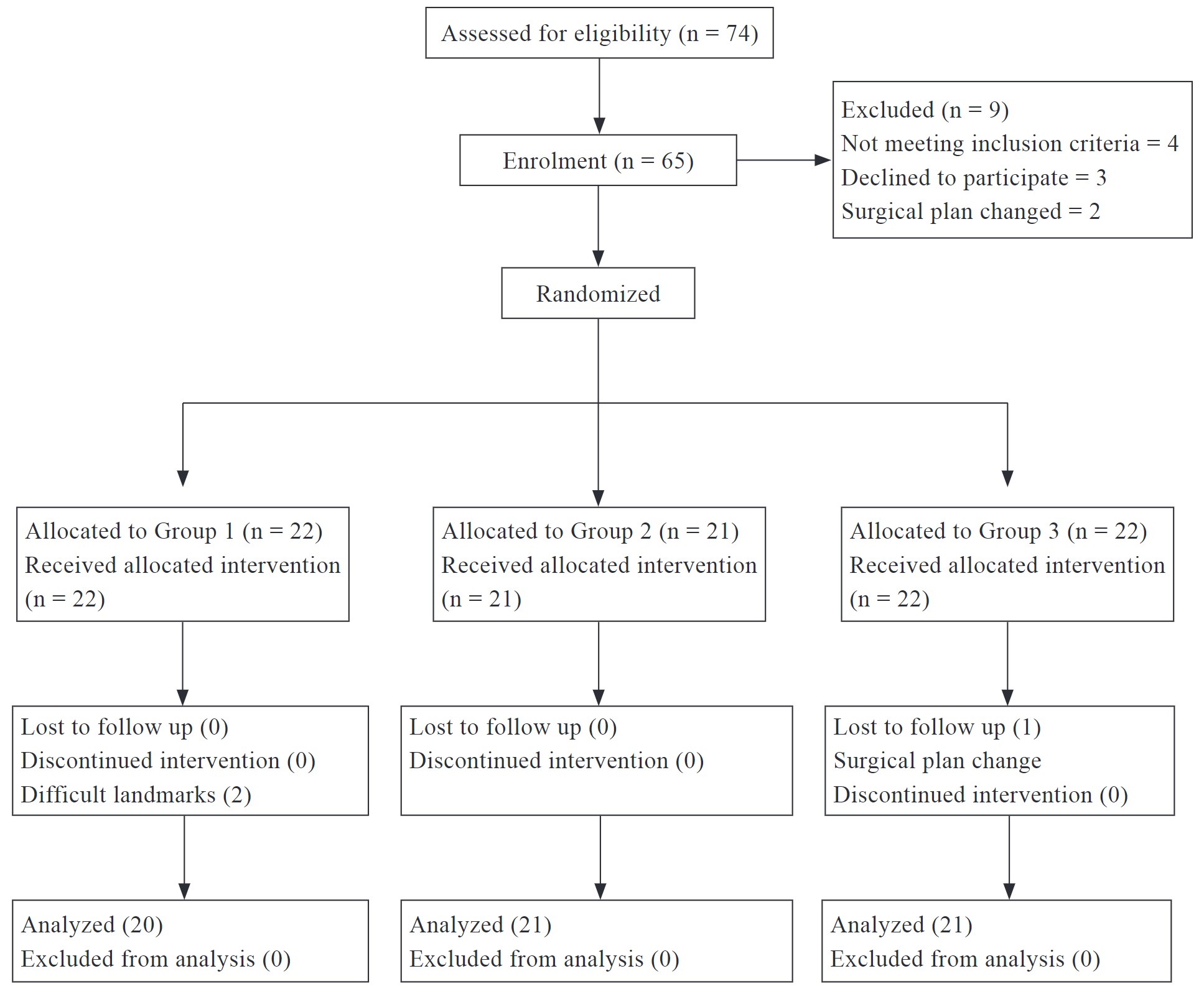
Download full-size image
Procedure
All patients underwent VATS wedge resection under general anesthesia. The patients in the DS group received 150 mg of DS into the gluteus maximus muscle under ultrasound guidance immediately after the induction of general anesthesia. Various analgesics were prescribed, as required, during the intraoperative and postoperative periods. Fentanyl, morphine, and nalbuphine were administered in the operating room or at the postanesthetic caring unit (PACU) at the discretion of the anesthesiologist. Other analgesic agents were prescribed in the general ward, as required. All patient received dexamethasone 5 mg and palonosetron 125 μg as prevention of postoperative nausea and vomiting (PONV).
Outcome Measurement
The primary endpoint was the effectiveness of pain relief between the two patient groups. We retrospectively analyzed total dosage of various analgesics prescribed during the intraoperative and postoperative periods. In addition, we converted opioid consumption during hospitalization into oral morphine equivalent dose. We also assessed the frequency of rescue analgesics in the general ward and conducted separate analyses based on whether the analgesics were opioids.
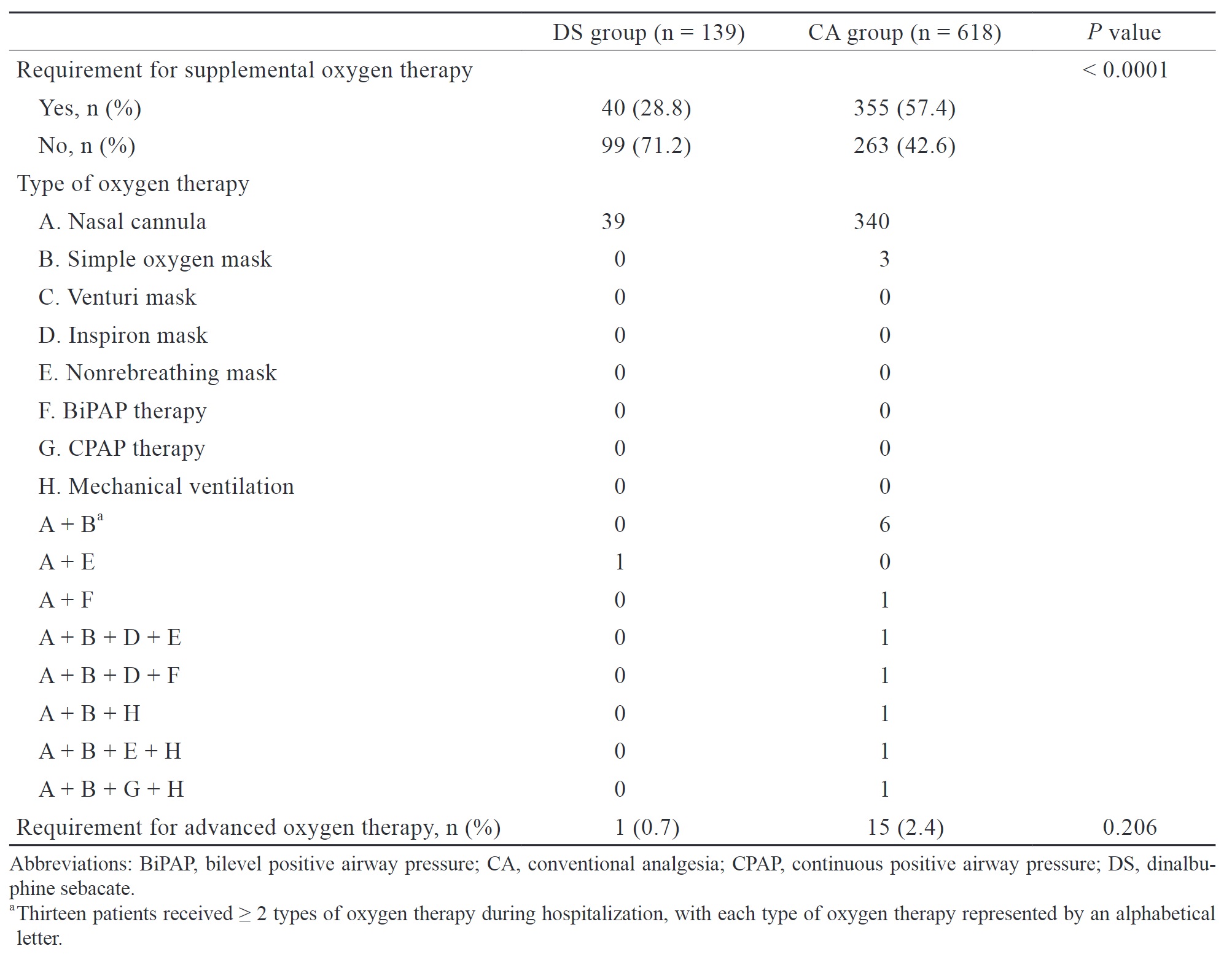
The secondary endpoint was the incidence of PPCs between the two patient groups. PPCs were defined based on the European perioperative clinical outcome definition, which includes respiratory infection, respiratory failure, pleural effusion, atelectasis, pneumothorax, bronchospasm, aspiration pneumonitis, and pneumonia. If the new International Classification of Diseases tenth revision (ICD-10) diagnostic code related to PPCs during hospitalization was present in medical records, it was considered a PPC caused by surgery. Additionally, we evaluated the use of supplemental oxygen during postoperative period. Advanced oxygen therapy was defined as receiving oxygen therapy at a higher flow rate or receiving oxygen therapy that is more invasive than a simple mask. The requirement for oxygen therapy may imply the occurrence of potential PPCs or respiratory depression induced by opioids.
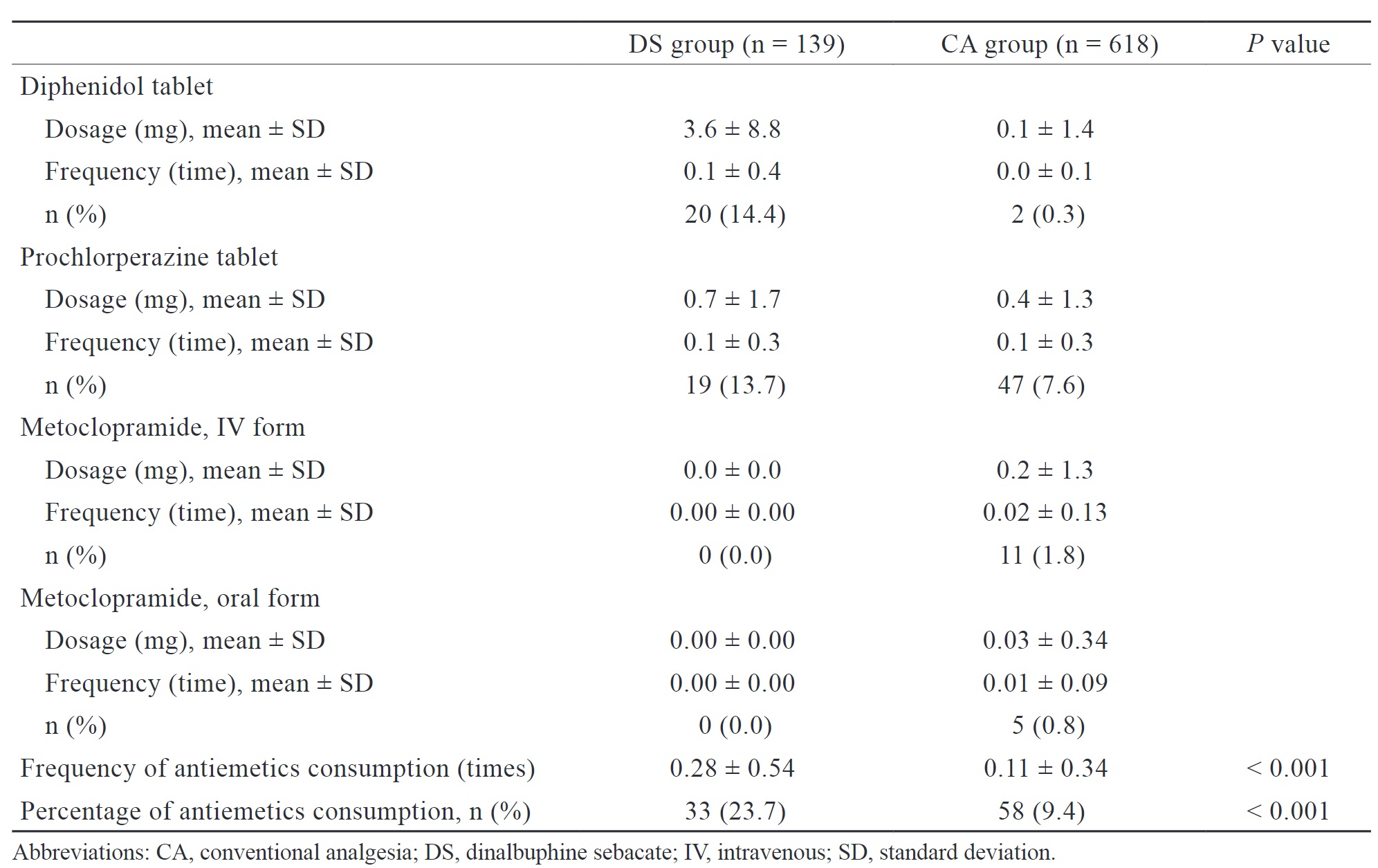
Nausea and vomiting are common adverse events associated with DS, and they may lead to PPCs. We assessed the percentage, frequency, and consumption of antiemetics during the postoperative period as a measurement of nausea and vomiting.
Statistical Analysis
Statistical analysis was conducted on data from a total of 757 patients. Descriptive statistics and Chi-square tests were used to analyze the clinicodemographic characteristics of patients. The primary outcomes were the frequency of rescue analgesics and the consumption of different analgesics. Differences between the two groups were analyzed by student’s T-test with a 95% confidence interval, and the statistical significance level was set at 0.05. The secondary outcomes included the incidence of PPCs and the use of supplemental oxygen therapy during postoperative period. As these outcomes were nominal scale data, contingency tables were constructed, and statistical testing was performed using the Chi-squared test or Fisher’s exact test, with a statistical significance level of 0.05. We also assessed the frequency of antiemetics consumption by patients during postoperative period, and differences between the two groups were analyzed using the Chi-squared test. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ clinicodemographic characteristics, including age, sex, BMI, length of hospital stay (LOS), and the CCI, are summarized in Table 1. The DS group had significantly shorter LOS (5.6 ± 2.3 vs. 6.8 ± 6.1 days,
Download full-size image
Download full-size image
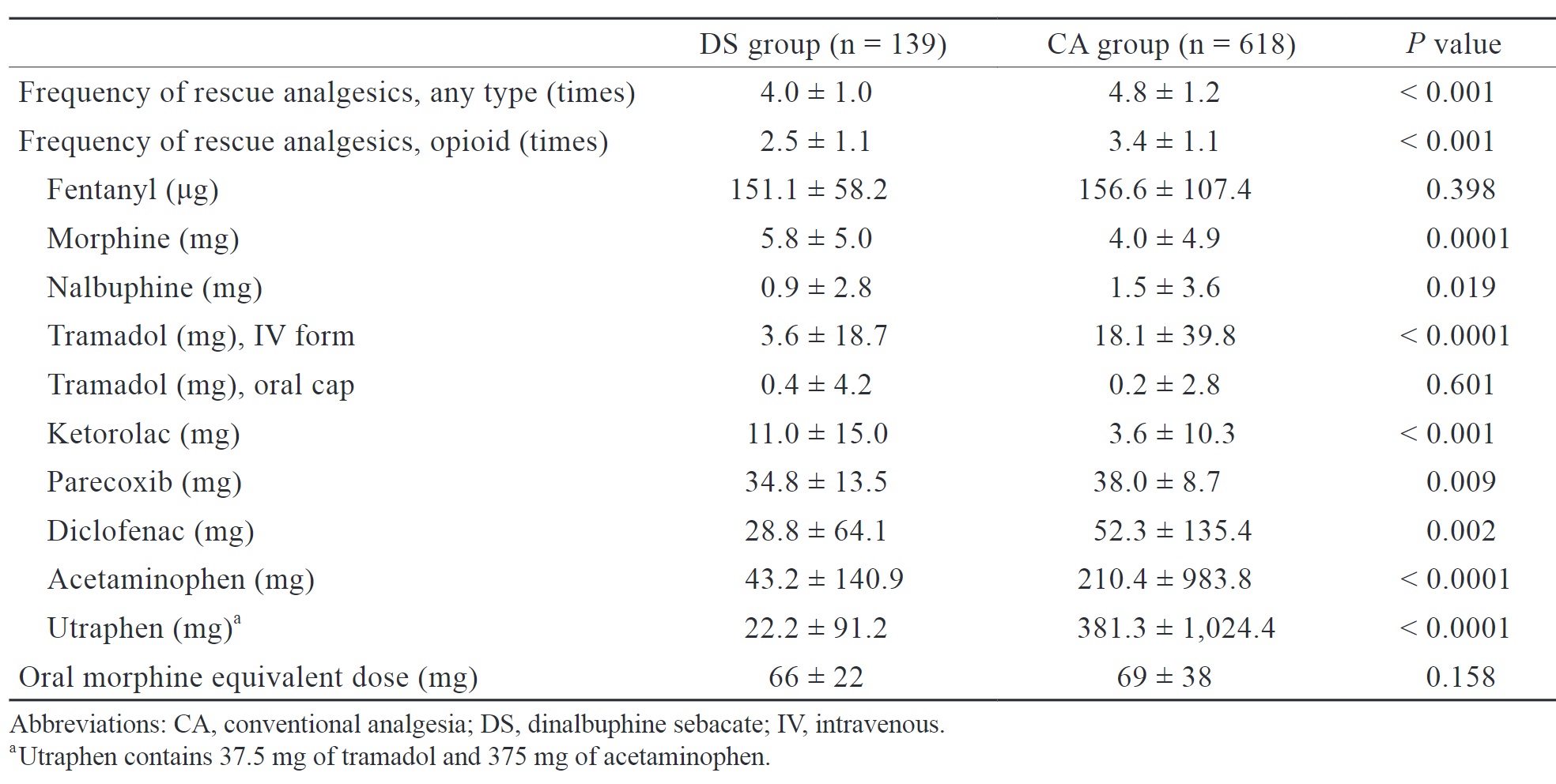
We retrospectively analyzed the frequency of analgesic use, use of opioids, cumulative dose of various analgesics, and oral morphine equivalent dose of opioid consumption during both intraoperative and postoperative period (Table 2). The DS group had a significantly lower frequency of analgesic use (4.0 ± 1.0 vs. 4.8 ± 1.2 times,
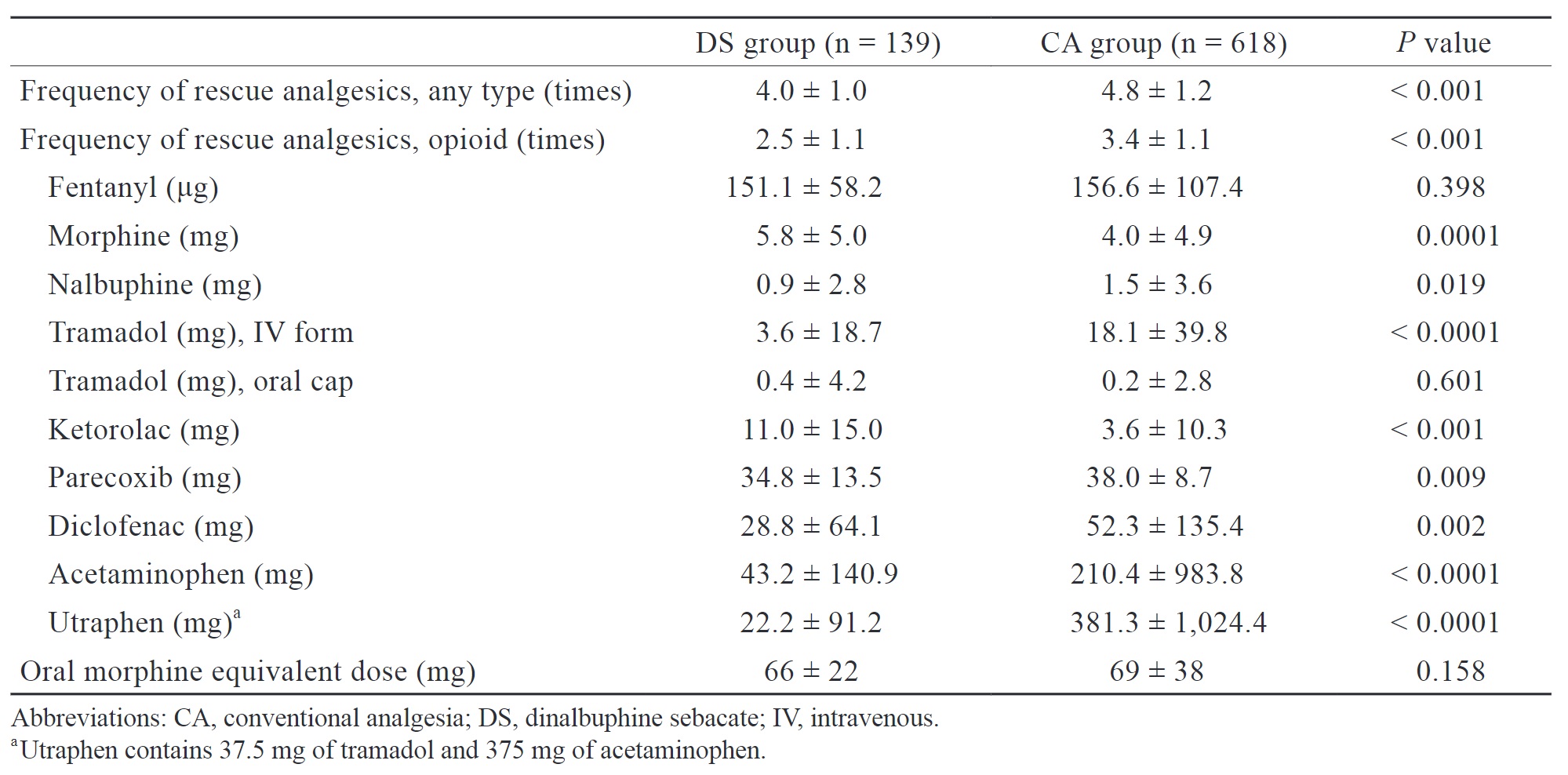
Download full-size image
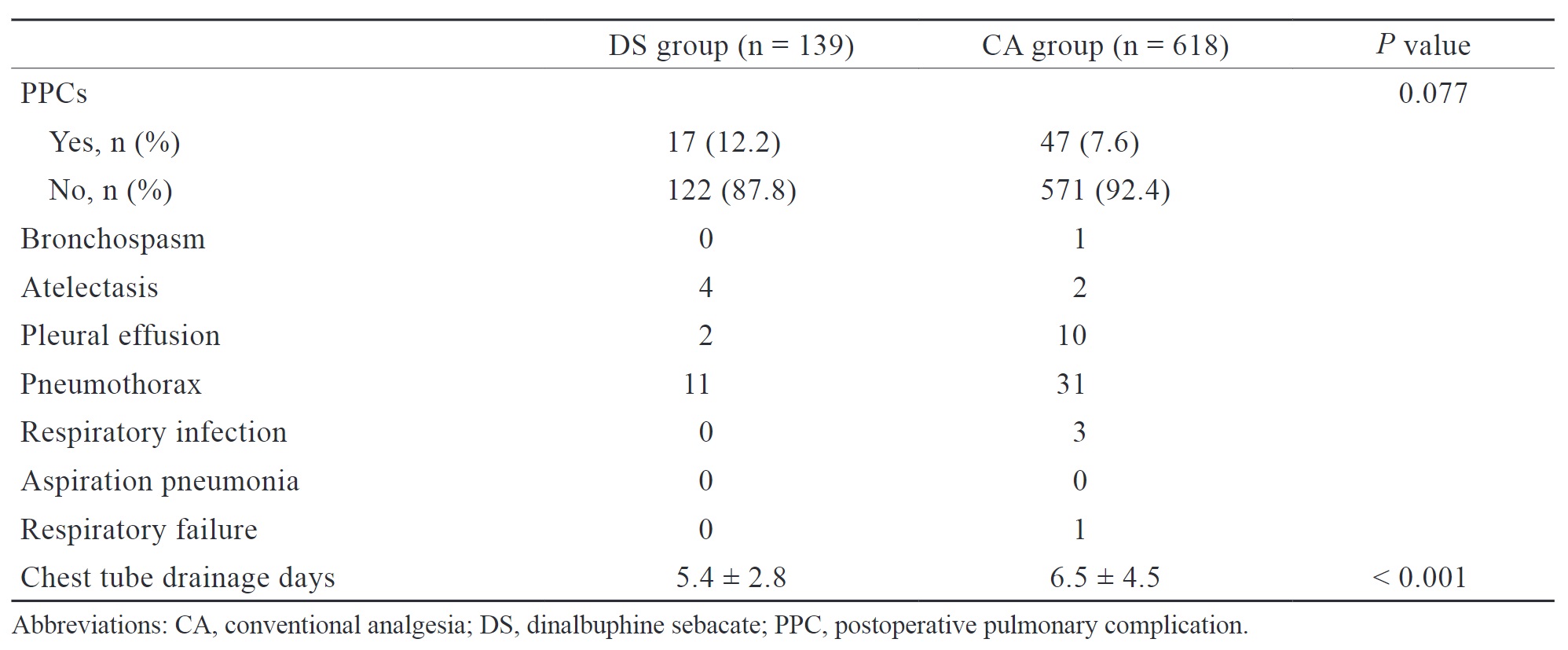
PPCs, including bronchospasm, atelectasis, pleural effusion, pneumothorax, respiratory infection, aspiration pneumonia, and respiratory failure, were defined and diagnosed based on any newly added ICD-10 code during hospitalization and are summarized in Table 3. No significant differences were observed in the incidence of PPCs between the DS and CA groups (12.2% vs. 7.6%,
Download full-size image
Download full-size image
The DS group had a significantly higher proportion of patients (23.7% vs. 9.4%,
Discussion
Download full-size image
As a part of MMA, DS is proved to be effective for relieving postoperative pain in various surgeries.23-28 Compared with CA, IV PCA, or placebo, patients taking DS experience significantly less postoperative pain, as measured using the numerical rating scale (NRS) or visual analog scale (VAS). The need for postoperative rescue analgesia varies depending on the type of surgery. The preoperative administration of DS was reported to reduce the requirement of rescue tramadol following upper limb fracture surgery.26 Similarly, patients undergoing hemorrhoidectomy who received DS significantly consumed less dose of rescue ketorolac both during hospitalization and after discharge.27 Both studies indicate that patients have significantly lower NRS or VAS scores postoperatively.
In our study, the DS group presented with a significantly lower frequency of rescue analgesic administration during hospitalization compared to the CA group. Additionally, although there were no statistically significant differences in oral morphine equivalent dose, the total consumption of certain analgesics, such as diclofenac, acetaminophen, and utraphen, throughout the entire hospitalization was lower in the DS group. This indirectly suggests that patients in the DS group still experienced a certain degree of long-lasting pain relief, as theoretically, patients experiencing less pain require less frequent analgesics administration. We attribute this result to the extended-release of nalbuphine after DS administration, resulting in a sustained concentration of nalbuphine in the plasma. When other rescue analgesics, except opioid, are administered, the presence of nalbuphine may result in a synergic effect on pain relief, thereby reducing the frequency of salvage analgesic use and the total consumption of certain analgesics in the DS group. Moreover, compared to conventional nalbuphine, DS significantly produces lower peak plasma concentrations of nalbuphine.22 We believe that this pharmacokinetic property allows DS to provide effective pain relief while inducing less sedation, nausea and vomiting compared to regular nalbuphine administration postoperatively.
Notably, compared with the CA group, when DS was administered, patients had higher morphine and ketorolac consumption but similar fentanyl consumption during hospitalization. This finding may also be attributed to the pharmacokinetics of DS. The peak serum concentration of nalbuphine in DS occurs approximately 64 hours after administration.22 Therefore, administering DS after anesthesia induction may not provide adequate analgesia for surgical stimuli. At our institution, fentanyl, morphine, nalbuphine, and ketorolac are commonly administered during surgery and in the PACU. Since nalbuphine does not reach peak serum concentration intraoperatively and immediately postoperatively to induce analgesic effects, the consumption of the above-mentioned analgesics did not reduce as a result. Administering DS earlier or increasing its dose may reduce intraoperative opioid use and achieve more effective analgesia. Future studies should determine the optimal timing and dose of DS administration.
The choice of analgesic technique and agent, such as gabapenoids, is associated with the incidence of PPCs. Given the potential risk, we aimed to evaluate the association between DS and the incidence of PPCs after VATS. The results showed a higher incidence of PPCs in the DS group (12.2% vs. 7.6%), but it is not statistically significant. Even though the DS group had a higher incidence of PPCs, the severity may be mild, as evidenced by the lower need for oxygen supplemental therapy, shorter duration of chest tube placement, and shorter LOS in our study. Due to the lack of laboratory data and computed tomography (CT) images as objective assessment tools, we indirectly evaluated the severity of PPCs based on whether patients required supplemental oxygen therapy. Theoretically, patients with no PPCs or only mild PPCs typically do not require supplemental oxygen therapy postoperatively.
Fernandez-Bustamante et al.29 reported that 33.4% of patients with ASA class 3 developed PPCs after noncardiothoracic surgery, with prolonged oxygen therapy through nasal cannula (19.6%) being the most common complication, followed by atelectasis (17.1%). Once a PPC developed, even if it was only associated with prolonged nasal cannula therapy, it was associated with an increased risk of early mortality, prolonged LOS, and a higher likelihood of admission to the intensive care unit. Therefore, the clinical significance of DS may lie in improving postoperative outcomes in these high-risk patient groups, even if it only reduces the duration of postoperative oxygen use, thereby enhancing their outcomes. PPCs can significantly impair patient outcomes; thus, efforts should be made to minimize the risk of PPCs.
Furthermore, oxygen use can lead to dry nasal mucosa and inconvenience for patients carrying oxygen delivery devices, thus reducing postoperative comfort. Therefore, regardless of whether supplemental oxygen administration is associated with postoperative outcomes, reducing supplemental oxygen therapy can at least improve patient comfort.
Patients in the DS group can have their chest tubes removed earlier. Thoracostomy tubes are associated with various complications, including malposition and infection. Studies have indicated an increased incidence of infection with prolonged tube or catheter placement.30 Thus, shortening the duration of thoracostomy tube indwelling can reduce the incidence of these complications. Recent research has shown that a shorter duration of thoracostomy tube placement is linked to improved outcomes, such as shorter LOS and lower pain scores.31 To the best of our knowledge, no clinical study has demonstrated the association between DS and PPCs. Our study is the first to investigate this association. Future prospective multicenter clinical trials are needed to confirm our findings.
Our study findings revealed that patients in the DS group had a higher total consumption of antiemetics and a more frequent antiemetic use postoperatively. This indirectly suggests that patients in the DS group may be more prone to experiencing dizziness, nausea, and vomiting, which is consistent with previous research. However, regardless of whether patients in the DS group received prophylactic or rescue antiemetics, the incidence of PPCs did not increase, and patients had a shorter LOS. Even if PONV occurred after DS administration, its severity is proved to be mild, and most of the cases are resolved within one day postoperatively without prolonged symptoms.23,25 Current research indicates that the occurrence of PONV after DS administration may be associated with patient characteristics and surgical procedures.28 An expert opinion and consensus on DS for perioperative management have been published in
Our study has several limitations. Firstly, it was a retrospective database analysis. Although we included all patients who underwent thoracoscopic wedge resection at our institution to reduce selection bias, our outcome measurement based on discharge medical records’ ICD-10 diagnosis codes is not the most objective assessment tool for PPCs diagnosis, leading to some degree of information bias. Secondly, there are many confounders that can affect the relationship between the intervention and the disease in the study. In the study design phase, we attempted to minimize the impact of confounders through restriction and matching. However, due to limitations in the database, such as the inability to extract more objective data like the actual timing and dosage of medication administration, it was challenging to use more advanced statistical methods, such as multivariate analyses and area under the curve, to eliminate the influence of confounders on the results. Finally, regarding outcome measurement, it was all evaluated indirectly. For example, data on the patients’ subjective evaluations of pain, such as through VAS or NRS, were not available in the database. Therefore, we could only indirectly assess the clinical effectiveness of DS based on analgesic consumption. The inferences are all indirect.
We recommend that future researchers keep utilizing subjective pain assessment tools such as VAS. Additionally, employing more objective assessment tools, such as laboratory data and CT images, for evaluating PPCs would be beneficial. Furthermore, tracking patients’ vital signs, oxygen therapy condition, and image-based lung collapse volume on a daily basis postoperatively can facilitate a more accurate clinical severity analysis.
Conclusion
The routine preoperative administration of DS as a component of MMA to patients undergoing VATS wedge resection can significantly reduce the hospitalization days, chest tube indwelling time, and various postoperative analgesic consumption. DS should be administered slightly earlier than just prior to incision for better intraoperative pain management; however, more research is required to determine the optimal timing of analgesic administration. Our data indicate that the incidence of PPCs was comparable between the two groups, but the DS group has less requirement of supplemental oxygen therapy in the ward, suggesting that DS can improve some patient outcomes. More prospective clinical studies, including randomized controlled trials, are necessary to verify and extend our findings.
References
| 1 |
Lawrence VA, Cornell JE, Smetana GW.
Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians.
Ann Intern Med. 2006;144(8):596-608.
|
| 2 |
Miskovic A, Lumb AB.
Postoperative pulmonary complications.
Br J Anaesth. 2017;118(3):317-334.
|
| 3 |
Agostini P, Lugg ST, Adams K, et al.
Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy.
Interact Cardiovasc Thorac Surg. 2017;24(6):931-937.
|
| 4 |
Aiolfi A, Nosotti M, Micheletto G, et al.
Pulmonary lobectomy for cancer: systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach.
Surgery. 2021;169(2):436-446.
|
| 5 |
Xie J, Wu Y, Wu C.
Is thoracoscopy superior to thoracotomy in the treatment of congenital lung malformations?
Ther Adv Respir Dis. 2020;14:1753466620980267.
|
| 6 |
Ye B, Wang M.
Video-assisted thoracoscopic surgery versus thoracotomy for non-small cell lung cancer: a meta-analysis.
Comb Chem High Throughput Screen. 2019;22(3):187-193.
|
| 7 |
Holleran TJ, Napolitano MA, Duggan JP, et al.
Predictors of 30-day pulmonary complications after video-assisted thoracoscopic surgery lobectomy.
Thorac Cardiovasc Surg. 2023;71(4):327-335.
|
| 8 |
Agostini PJ, Lugg ST, Adams K, et al.
Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy.
J Cardiothorac Surg. 2018;13(1):28.
|
| 9 |
Yang J, Xia Y, Yang Y, et al.
Risk factors for major adverse events of video-assisted thoracic surgery lobectomy for lung cancer.
Int J Med Sci. 2014;11(9):863-869.
|
| 10 |
Kaufmann K, Heinrich S.
Minimizing postoperative pulmonary complications in thoracic surgery patients.
Curr Opin Anaesthesiol. 2021;34(1):13-19.
|
| 11 |
Kehlet H.
Modification of responses to surgery by neural blockade: clinical implications.
|
| 12 |
Wu CL, Fleisher LA.
Outcomes research in regional anesthesia and analgesia.
Anesth Analg. 2000;91(5):1232-1242.
|
| 13 |
Ballantyne JC, Carr DB, deFerranti S, et al.
The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials.
Anesth Analg. 1998;86(3):598-612.
|
| 14 |
Wiebalck A, Brodner G, Van Aken H.
The effects of adding sufentanil to bupivacaine for postoperative patient-controlled epidural analgesia.
Anesth Analg. 1997;85(1):124-129.
|
| 15 |
Okamoto T, Matsuki Y, Ogata H, et al.
Association between averaged intraoperative nociceptive response index and postoperative complications after lung resection surgery.
Interact Cardiovasc Thorac Surg. 2022;35(6):ivac258.
|
| 16 |
Feray S, Lubach J, Joshi GP, Bonnet F, Van de Velde M; PROSPECT Working Group *of the European Society of Regional Anaesthesia and Pain Therapy.
PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations.
Anaesthesia. 2022;77(3):311-325.
|
| 17 |
Friedrich S, Raub D, Teja BJ, et al.
Effects of low-dose intraoperative fentanyl on postoperative respiratory complication rate: a pre-specified, retrospective analysis.
Br J Anaesth. 2019;122(6):e180-e188.
|
| 18 |
Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN.
Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression.
Anesth Analg. 2017;125(1):141-146.
|
| 19 |
Myhre M, Diep LM, Stubhaug A.
Pregabalin has analgesic, ventilatory, and cognitive effects in combination with remifentanil.
Anesthesiology. 2016;124(1):141-149.
|
| 20 |
Ohnuma T, Raghunathan K, Moore S, et al.
Dose-dependent association of gabapentinoids with pulmonary complications after total hip and knee arthroplasties.
J Bone Joint Surg Am. 2020;102(3):221-229.
|
| 21 |
Tan HS, Frere Z, Krishnamoorthy V, Ohnuma T, Raghunathan K, Habib AS.
Association of gabapentinoid utilization with postoperative pulmonary complications in gynecologic surgery: a retrospective cohort study.
Curr Med Res Opin. 2021;37(5):821-828.
|
| 22 |
Tien YE, Huang WC, Kuo HY, et al.
Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation.
Biopharm Drug Dispos. 2017;38(8):494-497.
|
| 23 |
Chang TK, Huang CW, Su WC, et al.
Extended-release dinalbuphine sebacate versus intravenous patient-controlled analgesia with fentanyl for postoperative moderate-to-severe pain: a randomized controlled trial.
Pain Ther. 2020;9(2):671-681.
|
| 24 |
Chang SH, Chang TC, Chen MY, Chen WC, Chou HH.
Comparison of the efficacy and safety of dinalbuphine sebacate, patient-controlled analgesia, and conventional analgesia after laparotomy for gynecologic cancers: a retrospective study.
J Pain Res. 2021;14:1763-1771.
|
| 25 |
Lee SO, Huang LP, Wong CS.
Preoperative administration of extended-release dinalbuphine sebacate compares with morphine for post-laparoscopic cholecystectomy pain management: a randomized study.
J Pain Res. 2020;13:2247-2253.
|
| 26 |
Zheng ZH, Yeh TT, Yeh CC, et al.
Multimodal analgesia with extended-release dinalbuphine sebacate for perioperative pain management in upper extremity trauma surgery: a retrospective comparative study.
Pain Ther. 2022;11(2):643-653.
|
| 27 |
Yeh CY, Jao SW, Chen JS, et al.
Sebacoyl dinalbuphine ester extended-release injection for long-acting analgesia: a multicenter, randomized, double-blind, and placebo-controlled study in hemorrhoidectomy patients.
Clin J Pain. 2017;33(5):429-434.
|
| 28 |
Lee YE, Wang SY, Chen JH, et al.
Efficacy and safety of parenteral injection of an extended release κ-receptor opioid sebacoyl dinalbuphine ester for acute and chronic pain after laparoscopic bariatric surgery: a randomized, placebo-controlled, double-blind trial.
Obes Surg. 2023;33(4):1192-1201.
|
| 29 |
Fernandez-Bustamante A, Frendl G, Sprung J, et al.
Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators.
JAMA Surg. 2017;152(2):157-166.
|
| 30 |
Maxwell RA, Campbell DJ, Fabian TC, et al.
Use of presumptive antibiotics following tube thoracostomy for traumatic hemopneumothorax in the prevention of empyema and pneumonia—a multi-center trial.
J Trauma. 2004;57(4):742-748.
|
| 31 |
Batchelor TJP.
Enhanced recovery after surgery and chest tube management.
J Thorac Dis. 2023;15(2):901-908.
|
| 32 |
Lee SO, Lu CH, Man KM, Cheng KI, Wong CS, Sun WZ.
Multimodal analgesia with extended-release dinalbuphine sebacate for perioperative management: expert opinion and consensus.
Asian J Anesthesiol. 2023;61(3):123-131.
|