Abstract
Infective endocarditis (IE) remains a rare yet critically severe condition, representing a considerable diagnostic challenge, especially among patients with pre-existing structural heart anomalies. This report details the clinical journal of a 49-year-old male with a known bicuspid aortic valve who initially exhibited nonspecific symptoms, leading to rapid clinical deterioration and the emergence of uncommon complications. The patient experienced an aortic root rupture and pericardial tamponade, necessitating urgent surgical intervention. Transesophageal echocardiography (TEE) was instrumental in confirming the diagnosis and facilitating the decision to perform a Bentall’s procedure. This care highlights the critical role of TEE in diagnosing complex cases of IE and the imperative for swift intervention.
Keywords
aortic root rupture, Bentall’s procedure, bicuspid aortic valve, infective endocarditis, transesophageal echocardiography
Introduction
Infective endocarditis (IE) is an uncommon yet serious condition with an estimation of 3.0–7.5 per 100,000 population.1 It is characterized by infection and inflammation of the endocardium, particularly affecting cardiac valves. Severe IE is associated with substantial morbidity and mortality rates and often requires surgical intervention.2 We present a compelling case of severe IE in a 49-year-old male, accentuating the rarity of complications, including aortic root rupture and pericardial tamponade. The case underscores the need to identify predisposing factors and emphasizes the complexities of managing IE.
Case Presentation
A 49-year-old male (height 157 cm, weight 57.2 kg, body mass index 23.2 kg/m2) with a history notable for hyperlipidemia and gastroesophageal reflux disease, previously diagnosed with a bicuspid aortic valve (BAV), sought medical attention due to intermittent chest tightness and fever. Despite initial nonspecific symptoms, the patient’s condition intensified, associated with syncope, unexplained cardiac tamponade, and loculated fluid along the ascending aortic and aortic arch revealed by initial transthoracic echocardiography and computed tomography angiography (CTA) led to a diagnostic pericardiocentesis revealed aortic valve vegetation (0.40 cm × 0.38 cm) and left chest hemothorax, prompting chest tube insertion, thoracoscopic decortication, and the creation of a pericardial-pleural window. Blood culture was positive for
Despite receiving standard medical treatment with penicillin G and subsequent ceftriaxone, the patient’s clinical symptoms persisted, leading to referral to our cardiac center. An emergent Bentall’s procedure was performed based on transesophageal echocardiography (TEE) findings, exposing calcified BAV leaflets, aortic valve vegetation, suspected aortic root rupture, and hematoma (Figure 1). During the operation, a moderate amount of pericardial tamponade with a massive hematoma covering the ruptured site was observed. Pulsatile outflow from the rupture site occurred upon hematoma removal, with no pulmonary artery trunk or right ventricular outflow tract injury. Aortic annulus reconstruction using a bovine graft and coronary artery bypass grafting addressed the left anterior descending (LAD) artery due to LAD septic embolism.
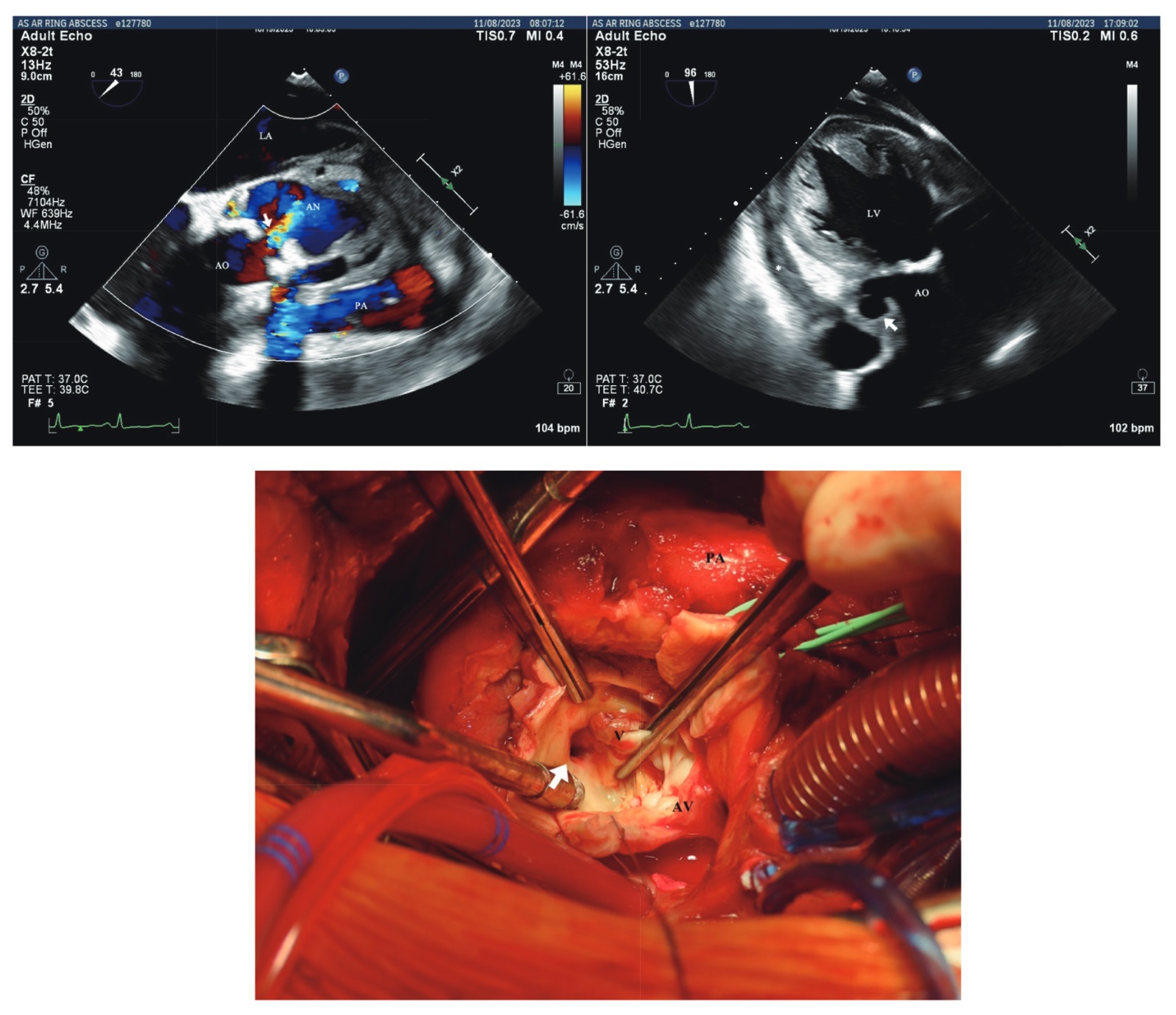
Download full-size image
Discussion
This case vividly illustrates the diagnostic intricacies associated with IE, particularly in the context of a patient with a BAV. The initial atypical symptoms and the historical context of the BAV underscore the difficulty in promptly recognizing abnormalities through conventional imaging modalities. The complexity is further heightened in the presence of cardiac tamponade and aortic root rupture, emphasizing the critical need for precise identification of these abnormalities. A noteworthy challenge in this case involved a hematoma mimicking a clot, creating difficulty in determining the cause of pericardial tamponade. Moreover, the timing of the CTA is crucial in this patient. The earlier CTA did not reveal paravalvular deformity. Given the dynamic nature of IE, and considering that paravalvular abscesses may develop during the clinical course, it may be necessary to repeat CTA to monitor for any evolving complications and guide appropriate clinical management.
Consequently, TEE emerged as a crucial diagnostic tool, showcasing its ability to navigate intricacies and unveil crucial details. TEE demonstrated superior temporal resolution, enabling the detection of small oscillating material and vegetation, and provided excellent evaluation of pseudoaneurysms and perforations.3 Additional color Doppler imaging proved instrumental in detecting small defects missed by valvular movement, especially when complicated relationships with adjacent structures were involved. The case revealed a normally positioned calcified BAV with small vegetation and mild to moderate aortic insufficiency. The paramount importance of evaluating pseudoaneurysms of the aortic root was emphasized, particularly the manifestation of small dimension openings with discernible color flow patterns on Doppler echocardiography. The distinctive pulsatile motion characterized by a pronounced thump, involving expansion during early systole followed by collapse during diastole, further delineated the diagnostic significance.
The presentation of perivalvular abscesses often manifested heterogeneous echogenicity of the aortic root. Rarely, the deteriorating aortic wall in both presentations may have led to the development of aortic root rupture. Doppler color flow imaging demonstrated potential worsening of aortic regurgitation and mosaic patterning at the mid-esophageal aortic valve short-axis view, signifying the necessity for surgery.
Accessing the true incidence of complications is challenging, given the often retrospective nature of cases. However, existing literature suggests that patients with BAV and IE are more prone to aortic root abscess and subsequent valve replacement (85%) compared to patients with Tricuspid Aortic Valve and IE (46%).4 Cases have revealed the extension of infection from the valve, leading to disruptions in the aortic wall and pseudoaneurysm-like structures. In rare instances, it culminates in the development of fistulas, aortic dissections, or myocardial perforations. This comprehensive analysis underscores the intricate diagnostic landscape associated with IE and highlights the indispensable role of advanced imaging techniques, particularly TEE, in navigating these complexities for accurate diagnosis and optimal patient management.5
Conclusion
This case emphasizes the inherent diagnostic challenges in identifying IE, particularly in patients with underlying structural heart disease. It underscores the critical role of TEE in diagnosing IE and guiding management decisions, especially in complex cases involving aortic root pathology. Timely diagnosis and intervention are pivotal to enhancing patient outcomes and mitigating the risk of potentially life-threatening complications associated with IE.
Acknowledgments
None.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent
Written informed consent was obtained from the patient for their anonymized information to be published in this article.
References
| 1 | |
| 2 |
Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography.
Recommendations for the practice of echocardiography in infective endocarditis.
Eur J Echocardiogr. 2010;11(2):202-219.
|
| 3 |
Kim IC, Chang S, Hong GR, et al.
Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and intracardiac complications in patients with infective endocarditis in the era of 3-dimensional images.
Circ Cardiovasc Imaging. 2018;11(3):e006986.
|
| 4 |
Kiyota Y, Della Corte A, Montiero Vieira V, et al.
Risk and outcomes of aortic valve endocarditis among patients with bicuspid and tricuspid aortic valves.
Open Heart. 2017;4(1):e000545.
|
| 5 |
Ryan EW, Bolger AF.
Transesophageal echocardiography (TEE) in the evaluation of infective endocarditis.
Cardiol Clin. 2000;18(4):773-787.
|