Abstract
Background
Spinal anesthesia-induced hypotension can lead to adverse consequences for the mother-fetus binomial. We compared two prophylactic phenylephrine infusions with placebo in obese patients during cesarean delivery (CD) under spinal anesthesia.
Methods
In this randomized, placebo-controlled, double-blind study, 121 patients were randomly allocated to receive 0.9% saline in Group C, prophylactic phenylephrine infusion 50 μg/min in Group P50, or prophylactic phenylephrine infusion 100 μg/min in Group P100, starting immediately after anesthesia induction until delivery. The primary outcome was the number of episodes of hypotension.
Results
The median (interquartile range) of the number of episodes of hypotension in Group P100 [0.0 (0.0–0.0)] and Group P50 [0.0 (0.0–1.5)] were lower in Group C [3.0 (2.0–5.0)],
Conclusion
Prophylactic phenylephrine infusion of 100 or 50 μg/min in obese women during CD under spinal anesthesia reduced the number of episodes of hypotension, the incidence of hypotension, the number of physician interventions, and the number of rescue phenylephrine boluses, and increased the time until the first hypotension episode. However, 100 μg/min could lead to more reactive hypertension.
Keywords
Apgar score, cesarean delivery, hypotension, obesity, phenylephrine, spinal anesthesia
Introduction
Spinal anesthesia is one of the most widely used techniques for cesarean delivery (CD) in obese women. 1 However, one of the most common undesirable events of spinal anesthesia is maternal hypotension, which can have potentially adverse consequences for the mother-fetus binomial. 2 Recently, studies have reported the association of increased body mass index (BMI) with a higher degree of spinal block, 3,4 and a higher incidence of hypotension 4-7 as well as the occurrence of a greater number of episodes of hypotension compared to pregnant women with normal BMI undergoing spinal anesthesia. Corroborating this finding, in an observational study involving 540 patients, we reported that an increase in BMI was associated with a higher sensory block level and a higher incidence of maternal hypotension. 8
Aortocaval compression by the uterus and venodilation, leading to decreased venous return and cardiac output, were previously believed to be the dominant mechanisms of hypotension after spinal anesthesia during CD. However, currently, a reduction in systemic vascular resistance (vasodilation of small arterioles) is the most important mechanism. 9 Therefore, using vasopressors, especially when used prophylactically, is imperative.
Phenylephrine, an α-1 receptor agonist, is a well-established agent used for the prevention and treatment of intraoperative hypotension in parturients undergoing CD. 10 However, there is still a lack of studies evaluating phenylephrine doses in CD of obese women.
A clinical trial carried out by George et al. 11 compared the use of prophylactic phenylephrine infusion (50 μg/min) with bolus dosing in obese parturients. However, in their study, the primary outcome was the incidence of intraoperative nausea and vomiting, which is different from the purpose of the current study.
To our knowledge, this study is the first randomized, placebo-controlled clinical trial that evaluated the use of two different infusions of phenylephrine compared to placebo in obese parturients for the prevention of hypotension during CD.
The objective of this study was to compare two prophylactic phenylephrine infusions with a placebo for the prevention of hypotension in obese patients during CD undergoing spinal anesthesia.
Methods
Subjects and Ethics
This parallel, randomized, placebo-controlled, clinical trial was carried out at Júlio Müller Hospital of the Federal University of Mato Grosso, Brazil after approval from the Research Ethics Committee (CAAE: 89114418.2.0000.5541). The trial was registered in the Brazilian Clinical Trials database ( https://ensaiosclinicos.gov.br/rg/RBR-42dch5 ). We obtained written informed consent from all patients. This study followed the CONSORT guidelines. Our institution is a tertiary hospital that serves women with high-risk pregnancies.
Inclusion Criteria and Exclusion Criteria
Between July 2018 and August 2022 (there was a considerable impact in recruiting patients for the study during the COVID-19 pandemic), pregnant women with American Society of Anesthesiologists physical status II–III and BMI ≥ 30 kg.m -2 undergoing elective CD under spinal anesthesia were eligible to participate in this study. The exclusion criteria included age < 18 years old, gestational age < 37 weeks, pregnancy with twins, labor, preeclampsia, chronic hypertension with arterial blood pressure ≥ 140/90 mmHg, New York Heart Association functional class > II, cerebrovascular disease, intrauterine growth retardation, fetal malformations and contraindications to spinal anesthesia.
Randomization Assignment
Online random number software ( www.graphpad.com ) was run by the principal investigator to generate a computer-generated sequence of codes (in a ratio of 1:1:1) that were placed into sealed, opaque envelopes. Each envelope included instructions for preparing the drug. The patients were randomized to receive 0.9% saline in Group C, phenylephrine 50 μg/min in Group P50, or phenylephrine 100 μg/min in Group P100. In Group C, the infusion contained 100 mL of 0.9% saline. In Groups P50 and P100, the infusion contained 100 mL of 0.9% saline plus 5 mg (50 μg/mL) and 10 mg (100 μg/mL) of phenylephrine, respectively. A nurse (2 different nurses participated in this process) who was not directly participating in patient care or involved in the study opened the sealed opaque envelopes containing the groups, prepared the solutions, and gave them to the anesthesiologist. All infusions were administered using a volumetric infusion pump at a flow rate of 60 mL/h immediately after the completion of the spinal anesthetic injection until delivery. Patients, anesthesiologists, surgeons, and pediatricians were blinded to the patients’ group assignments. All anesthesiologists were aware of the standard protocol of the study. An observer who was blinded to the groups recorded the study data.
Anesthesia and Operative Technique
In the operating room, patients were monitored with an electrocardiogram, noninvasive blood pressure suitable for each patient’s arm circumference, and pulse oximetry. Three readings of blood pressure and heart rate (HR) were recorded with the patient in the sitting position, and the average of these readings was considered the baseline value.
An 18 G-intravenous (IV) cannula was inserted. Then, 500 mL of lactated Ringer’s solution, followed by 10 mL/kg/h, was administered with an infusion pump until the end of the operation. Additionally, 2 g of cefazolin was administered before subarachnoid puncture. Spinal anesthesia was performed with the patient in the sitting position using a 25-G × 9-mm or 25-G × 12-mm Quincke needle, preferentially at the L3–4 vertebral interspace. Hyperbaric bupivacaine solution 12.5 mg (2.5 mL) was administered into the subarachnoid space at a rate of 1 mL every 10–15 seconds, followed by morphine 100 μg plus fentanyl 15 μg. Then, the patient was placed in the supine position, and the operating table was tilted 15° to the left. The sensory block was assessed by loss of pain sensation. Surgery was allowed if the level of sensory block was at least at the T6 dermatome. All patients received indwelling bladder catheterization. The operation was performed using the Pfannenstiel incision without exteriorization of the uterus.
Blood pressure and HR were recorded every 2 minutes before delivery and at the fifth minute after delivery. Hypotension, defined as a systolic blood pressure (SBP) < 100 mmHg or < 20% of baseline, was treated with a bolus of phenylephrine 0.1 mg IV. Reactive hypertension, defined as an SBP > 20% of baseline, was treated by stopping the infusion. The infusion was restarted if SBP returned to values less than 120% of the baseline. Bradycardia, defined as an HR < 50 beats/min, was treated with atropine 0.5 mg IV. Nausea or vomiting unrelated to arterial hypotension was treated with ondansetron 4 mg IV.
Immediately after delivery, 3 IU of oxytocin were administered as a bolus over 5 seconds, followed by 12 IU as a slow infusion. Oxygen 3 L.min -1 was administered by nasal catheter if the pulse oximetry reading was < 95% or if patients complained of shortness of breath.
Outcome Variables
Data were collected regarding the following variables: age, weight, height, BMI, comorbidities, gestational age, parity, previous CD, fasting time, sensory block level reached 15 minutes after subarachnoid injection, the interval of time from the spinal drug injection to the skin incision (spinal-to-incision interval), the interval of time from skin incision to delivery (incision-to-delivery interval), total operative time, fluids infused during surgery, and birth weight.
The primary outcome of the study was the number of episodes of hypotension.
Secondary outcomes were the incidence of hypotension, time to the first episode of hypotension, the incidence of reactive hypertension, the incidence of bradycardia, the number of physician interventions (stopping or restarting phenylephrine infusion, administering rescue phenylephrine, and administering atropine), and the incidence of nausea and vomiting. Finally, the incidence of Apgar scores < 7 at the first and fifth minute and pH, pO 2 , pCO 2 , base excess values of the umbilical artery, and headache, dizziness, neck pain, or any other side effects in the parturient were also not recorded.
Sample Size Calculation and Data Analysis
The sample size calculation was based on the mean ± standard deviation (SD) of 7.31 (3.97) of the number of episodes of hypotension in obese women undergoing CD reported by Nani and Torres. 12 A minimum of 32 patients were required to detect a difference of 2.92 (40% reduction) in the number of episodes of hypotension, considering an α error of 5% and a β error of 20%. Allowing for potential dropouts, the expected sample size was set at 42 patients per group.
Values were expressed as mean ± SD, median and interquartile range (IQR), or number of patients (%). Normality tests were performed using the Kolmogorov–Smirnov test. Continuous data with a normal distribution were analyzed by analysis of variance (ANOVA) followed by Tukey’s test for post hoc analysis if necessary. Discrete or continuous data without a normal distribution and ordinal data were analyzed using the Kruskal–Wallis test, followed by the Mann–Whitney test for post hoc analysis if necessary. For categorical data, the chi-square test was used to detect differences among groups, and Fisher’s exact test was used to detect the differences within groups. Kaplan–Meier curves were constructed for the time variable of the first episode of hypotension, and the differences between groups were analyzed by the log-rank test. A generalized estimation equation model was used for a comparative analysis of the evolution of the variables of SBP and HR over time among groups. The significance level for overall tests of group differences was 0.05. Adjustment for pairwise group comparisons was performed using Bonferroni’s test by dividing the significance level of 0.05 by the number of comparisons, yielding a corrected significance level of 0.017. Analyses were performed using the Statistical Package for Social Sciences software, version 21, and MedCalc software, version 20.
Results
One hundred forty-five patients were assessed for eligibility. One hundred twenty-six patients were randomized to the study. Two patients were excluded due to failed spinal anesthesia, two patients were excluded due to infusion pump malfunction, and another was excluded due to temporary loss of venous access. A total of 121 parturients completed the study and were included in the final analysis. The flow diagram of eligibility, allocation, follow-up, patient analysis, and causes of exclusion is shown in Figure 1.
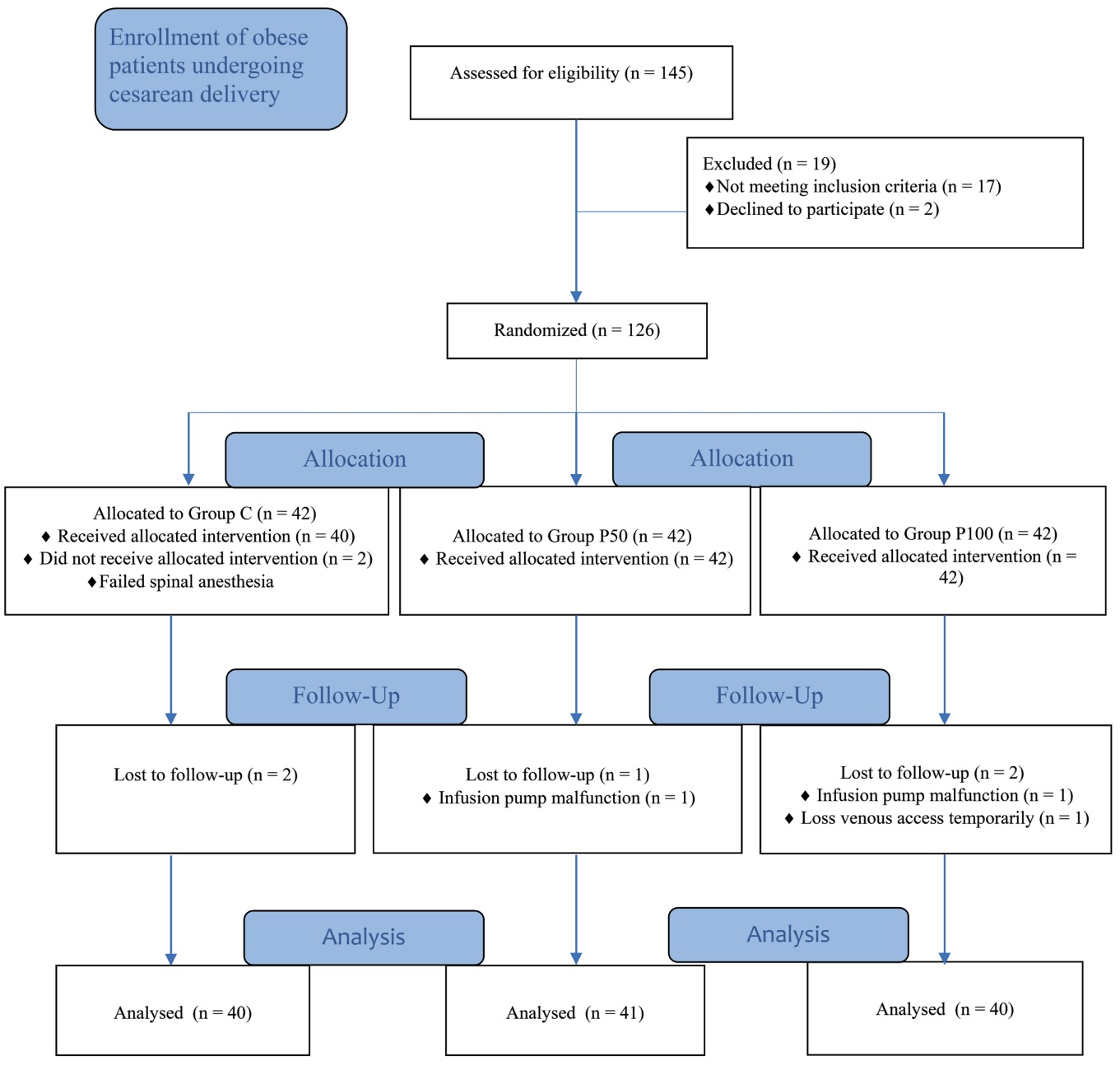
Download full-size image
There were no significant differences in patient demographic characteristics, fasting, extent of sensory blockade, spinal-to-incision interval, incision-to-delivery interval, total operative time, birth weight, or fluids infused during surgery (Table 1).
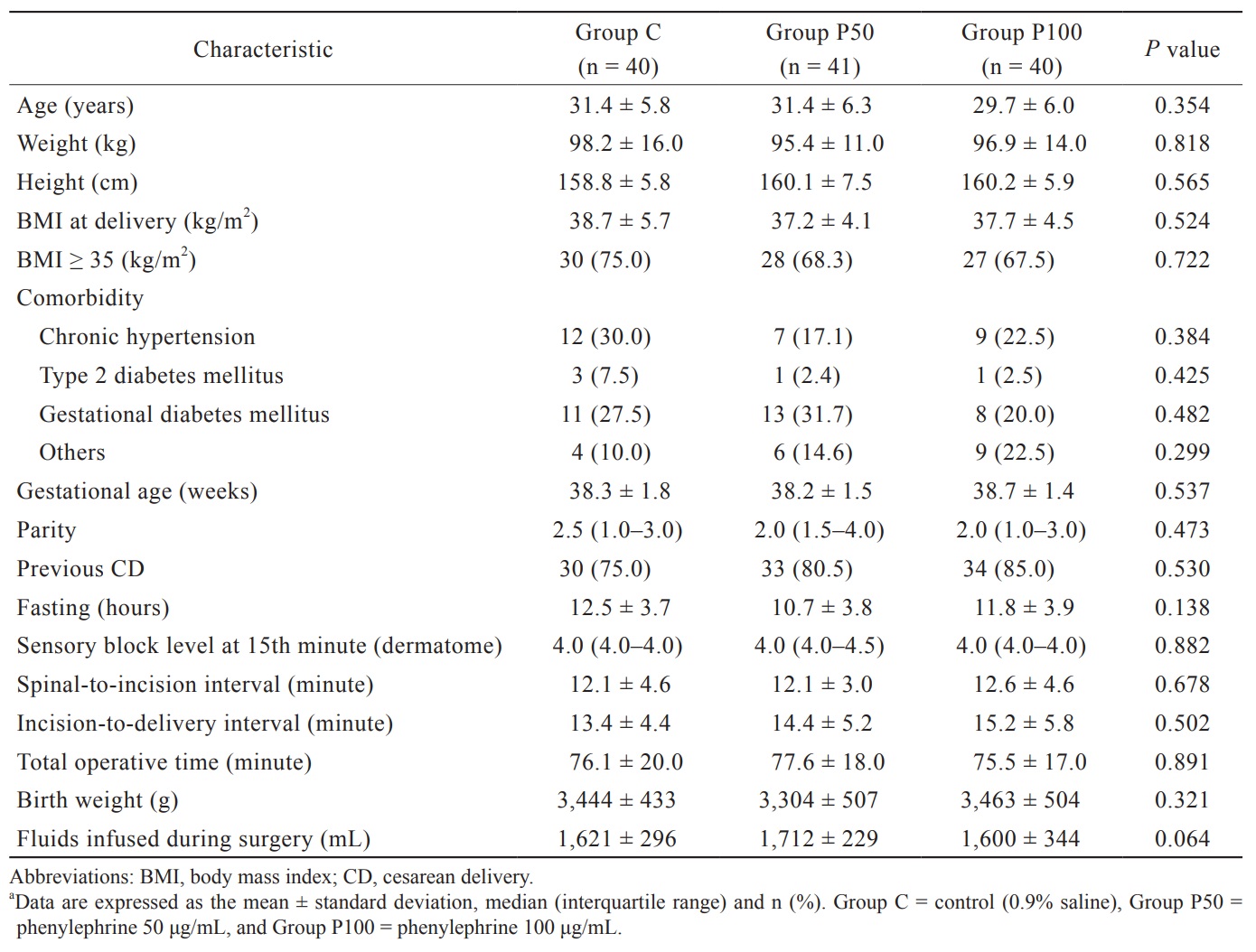
Download full-size image
The median (IQR) of the number of episodes of hypotension in Group P100 [0.0 (0.0–0.0)] and Group P50 [0.0 (0.0–1.5)] were threefold lower than those in Group C [3.0 (2.0–5.0)],
The incidence of hypotension was lower in Group P100 (10.0%) and Group P50 (29.3%) than in Group C (85.0%;
The incidence of reactive hypertension was higher in Group P100 (40.0%) than in Group C (7.5%;
The median (IQR) of the number of physician interventions needed to maintain maternal hemodynamics within the target range in Group P100 [0.5 (0.0–2.0)] and Group P50 [0.0 (0.0–2.5) was lower compared to Group C [3.5 (2.2–5.0),
There were no significant differences in the incidence of bradycardia, nausea, or vomiting among the groups.
Apgar scores < 7 at the first and fifth minutes and the values of pH, pO 2 , pCO2, and base excess of the umbilical artery were not different among the groups. Obstetric and neonatal outcomes are presented in Table 2.
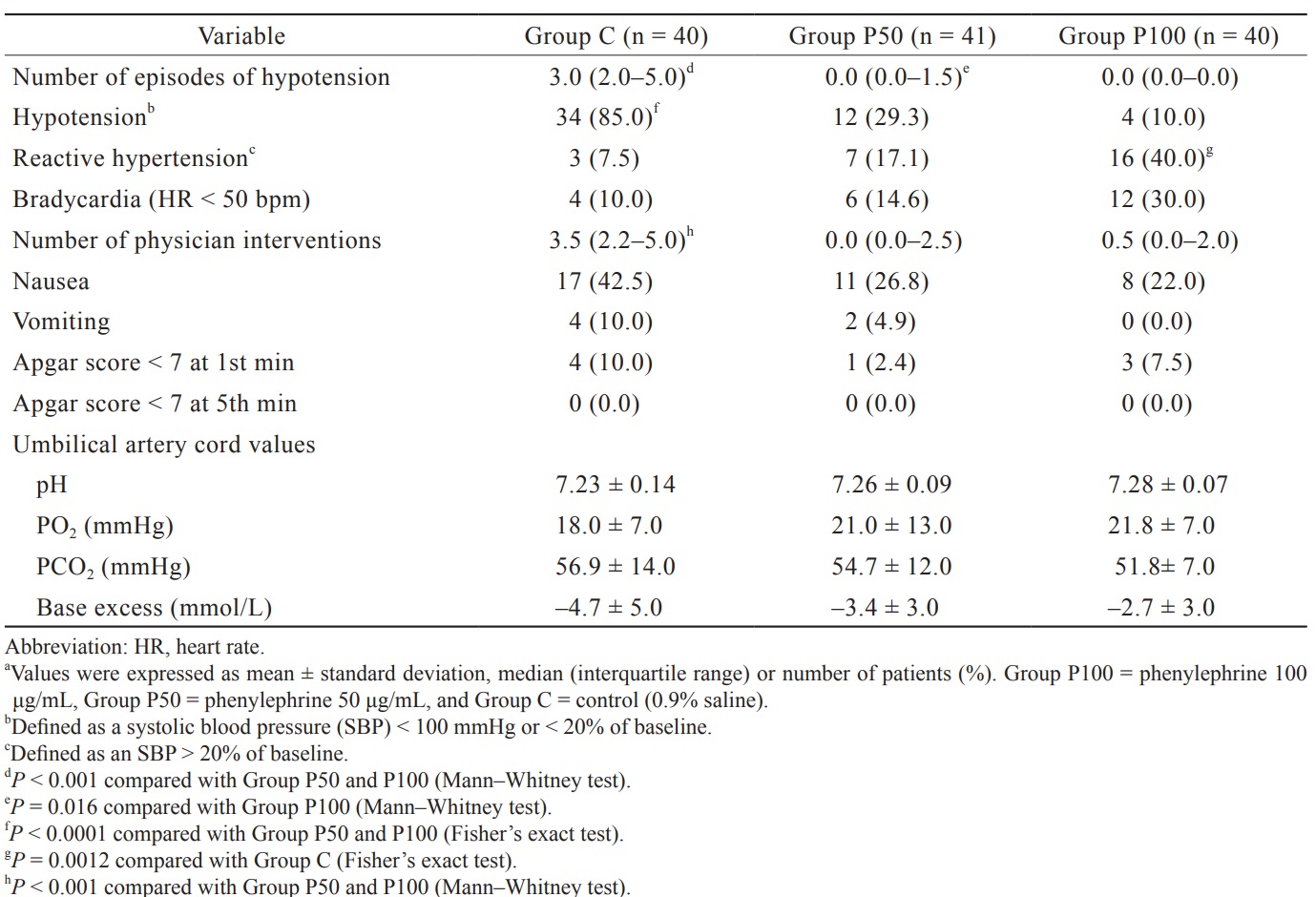
Download full-size image
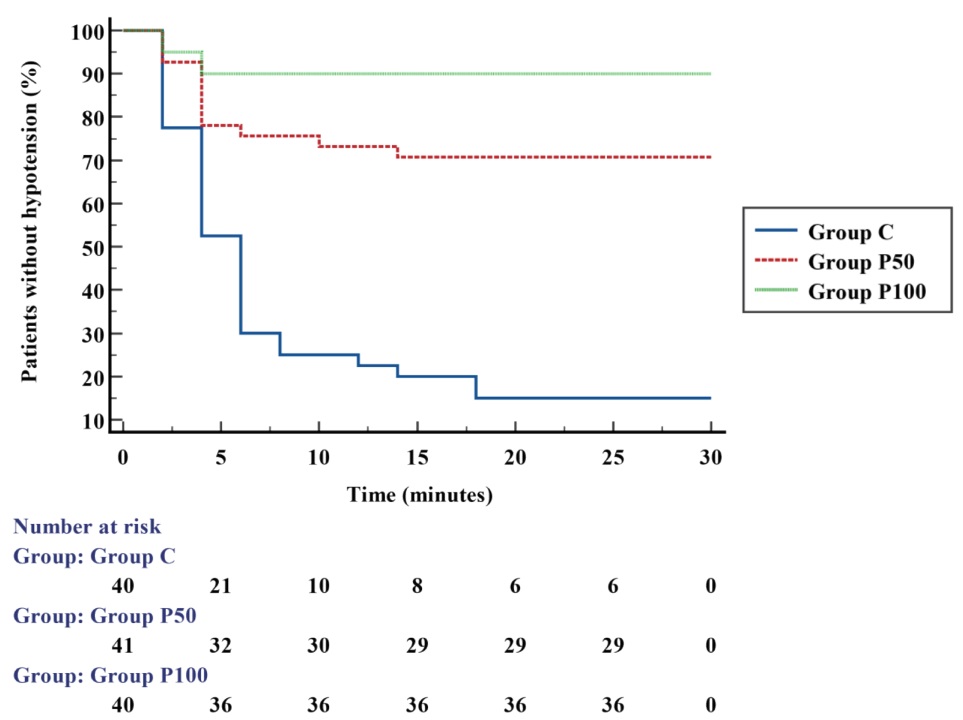
The time to the first episode of hypotension was significantly increased in Group P100 and Group P50 compared to Group C (
Download full-size image
SBP was higher over time in Group P100 and Group P50 than in Group C (
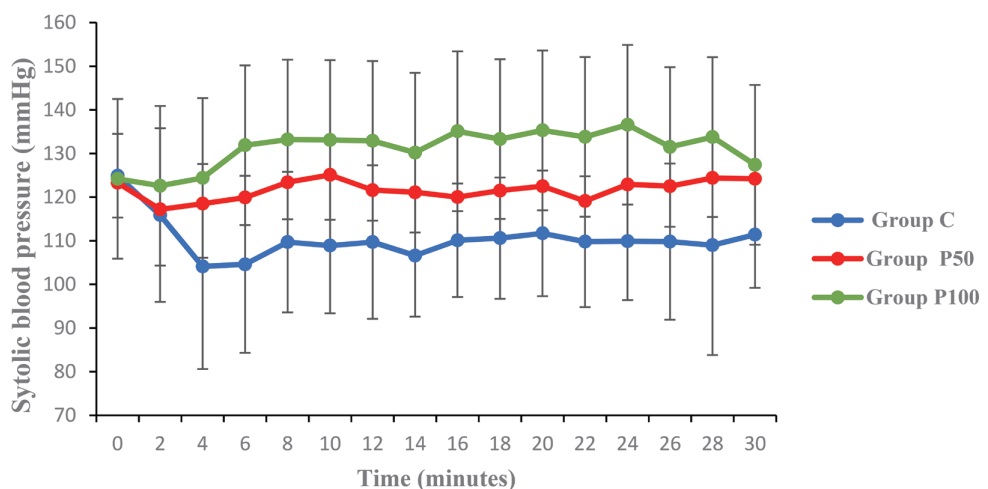
Download full-size image
Download full-size image
Discussion
In this randomized, placebo-controlled clinical trial, two doses of prophylactic phenylephrine infusion were superior to placebo for the prevention of intraoperative maternal hypotension in obese undergoing elective cesarean under spinal anesthesia.
Phenylephrine infusion to prevent maternal hypotension during CD is currently recommended as a first-line vasoactive drug by the latest expert consensus. 10 However, this recommendation was extracted from studies that excluded patients with a BMI ≥ 30 kg/m 2 . Thus, the prophylactic dose of phenylephrine appropriate for obese parturients has not been established.
Recently, Yang et al. 13 using Dixon and Massey’s up-down sequential method, reported that the 50% effective dose of phenylephrine needed to prevent hypotension after spinal injection was 21.92 μg/min (95% confidence interval [CI], 14.90–28.94) in non-obese pregnant women and 42.14 μg/min (95% CI, 24.58–59.70) in obese pregnant women with a ratio of relative potency of obese to non-obese pregnant women of 1.92 (95% CI, 1.09–3.14). The results suggest the need for higher doses of phenylephrine in obese pregnant women undergoing CD, as used in the present study.
A dose of 12.5 mg of hyperbaric bupivacaine associated with 0.1 mg of morphine has been used routinely in spinal anesthesia for CD in our service. Lee et al. 14 reported that the 95% effective dose and the 95% CI of hyperbaric bupivacaine, obtained through the up-down technique of modified sequential location, were similar for eutrophic and obese women (12.78 mg [95% CI, 10.75 to + infinity] and 11.86 mg [95% CI, 11.31 to 15.61], respectively).
Hypotension, in this study, was defined as an SBP < 100 mmHg or < 20% of baseline. However, definitions varied between those using an absolute blood pressure value, ranging from 80 to 100 mmHg, and a decrease from 0% to 30% of baseline or a combination of an absolute value and a percentage decrease, which could reflect significant differences between the results of studies.
The main result of the study was the reduction in hypotension episodes [median (IQR)] in the groups that received phenylephrine infusion at a dose of 50 μg/min [0.0 (0.0–1.0)] and 100 μg/min [0.0 (0.0–0.0)] when compared to placebo [3.0 (2.0–5.0)]. These results are similar to the study of George et al. 11 , who performed a multicenter, double-blind, randomized, placebo-controlled trial in 160 obese women with current BMI > 35–55 kg/m 2 undergoing spinal anesthesia with hyperbaric 0.75% bupivacaine 12 mg, in which hypotension was defined as SBP < 80% of baseline or < 90 mmHg. They reported a median (IQR) [0.0 (0.0–1.0)] of episodes of hypotension with the use of phenylephrine infusion of 50 μg/min compared with patients who received a single rescue dose of phenylephrine for treatment of hypotension [3.0 (1.0–4.0)]. Additionally, in the study by Allen et al. 15 , which included patients with BMI < 45 kg/m 2 , the hypotension episodes observed in the group of patients who received phenylephrine infusions of 100 μg/min were similar to those in our study, with median (IQR) of 0.0 (0.0–0.0).
In the present study, the incidences of hypotension were 10.0% and 29.3% in the patients who received phenylephrine infusion at rates of 100 and 50 μg/min, respectively. This outcome is consistent with the findings of studies in non-obese 16-18 and obese patients. 11
However, some studies have reported very low or even no hypotension with the use of phenylephrine infusion at a rate of 50 μg/min. In one of these studies, a lower dose of hyperbaric bupivacaine (10 mg) was used, and the incidence of hypotension was reported to be 2.5%. 19 In another study, 20 mL/kg hydroxyethyl starch (limited to 1 L) was infused until delivery, and no maternal hypotension was reported. 20 This absence of hypotensive events is perhaps the result of using a high dose of hydroxyethyl starch. However, there are concerns associated with colloid use in pregnant women. 21
The Kaplan–Meier curves showed that in patients who received phenylephrine infusions, the first episode of hypotension occurred mostly within the first four minutes, which could demonstrate the need to administer a bolus of phenylephrine immediately prior to initiation of a fixed rate after spinal anesthesia to around the delay in achieving effective blood levels, as reported by Kuhn et al. 22
The incidence of reactive hypertension in the present study was only higher in patients receiving phenylephrine at a dose of 100 μg/min when compared with 0.9% saline (40.0% vs. 7.5%,
Stopping or restarting phenylephrine infusion, administration of a phenylephrine bolus to treat hypotension, and administering atropine typically require multiple interventions from the anesthesia provider, which can be laborious and time-consuming. In this study, phenylephrine infusions were associated with fewer provider interventions to maintain hemodynamic stability compared to 0.9% saline, comparable to findings in pregnant women with a higher BMI. 11
In the present study, there was no significant difference among the groups regarding the incidence of bradycardia. This result was similar to those found in the study by George et al. 11 who also studied obese patients. According to a recent meta-analysis, phenylephrine was related to a higher incidence of bradycardia; however, no clinical trials reported hemodynamic instability associated with bradycardia. 23 Nevertheless, in the comparative analysis of the evolution of HR over time among groups, a significantly lower HR was observed in the groups that received phenylephrine infusion compared with 0.9% saline (Figure 4).
Hypotension, intraoperative pain, and medications, such as uterotonics and antibiotics, are among the main causes of nausea and vomiting during CD. 24 Studies have shown a reduction in nausea and vomiting with the use of phenylephrine in parturients. 11,25,26 However, our study did not show a significant association between the doses of phenylephrine infusion and the incidence of nausea and vomiting, which could be explained by the study being underpowered for this outcome.
The use of phenylephrine infusion proved to be safe for neonates, in terms of both Apgar score 13,15-18 and umbilical cord values. 15-17 Furthermore, a higher dose of phenylephrine infusion compared to a lower dose can improve the pH of neonates, as demonstrated by Ngan Kee et al. 27
Our present study has limitations. First, BMI ≥ 30 kg/m 2 at delivery, used as a criterion to define obesity, is subject to criticism since it might not reflect obesity in fact. It would be better to use as a criterion for obesity prepregnancy BMI ≥ 30 kg/m 2 or predetermined pregnancy-specific cutoff values for BMI (World Health Organization classes + 5 kg/m 2 ) for women at the time of delivery, as in the study by Dennis et al. 28 However, this limitation did not affect the extrapolation of our results since the average BMI of all groups was greater than 35 kg/m 2 . Second, we did not use noninvasive cardiac output measurements based on impedance cardiography or transthoracic echocardiogram to assess the hemodynamic impacts of the different phenylephrine infusions in the patients. Third, a larger sample size could bring more normalized variables and give the test more power, particularly in demonstrating differences between doses of 50 and 100 μg/min.
In conclusion, we found that intraoperative administration of phenylephrine infusion of 50 μg/min or 100 μg/min in obese women during CD under spinal anesthesia with 12.5 mg of hyperbaric bupivacaine reduced the number of episodes of hypotension, the incidence of hypotension, the number of physician interventions, and increased the time until the first hypotension episode. However, 100 μg/min could lead to more reactive hypertension. Nausea and vomiting, bradycardia, and neonatal outcomes were not different among the groups. More studies should be performed to evaluate the anti-hypotensive effects of different doses of phenylephrine infusion with or without the use of bolus doses prior to phenylephrine infusion in this population.
References
| 1 |
Taylor CR, Dominguez JE, Habib AS.
Obesity and obstetric anesthesia: current insights.
Local Reg Anesth. 2019;12:111-124.
|
| 2 |
Massoth C, Töpel L, Wenk M.
Hypotension after spinal anesthesia for cesarean section: how to approach the iatrogenic sympathectomy.
Curr Opin Anaesthesiol. 2020;33(3):291-298.
|
| 3 |
Lamon AM, Einhorn LM, Cooter M, Habib AS.
The impact of body mass index on the risk of high spinal block in parturients undergoing cesarean delivery: a retrospective cohort study.
J Anesth. 2017;31(4):552-558.
|
| 4 |
Elmeliegy M.
Effect of body mass index on anesthesia characteristics and vasopressor requirements during spinal anesthesia for elective cesarean section.
Open J Anesthesiol. 2020;10(4):157-169.
|
| 5 |
Fakherpour A, Ghaem H, Fattahi Z, Zaree S.
Maternal and anaesthesia-related risk factors and incidence of spinal anaesthesia-induced hypotension in elective caesarean section: a multinomial logistic regression.
Indian J Anaesth. 2018;62(1):36-46.
|
| 6 |
Liu Y, Qian Y.
Analysis of risk factors for intraoperative hypotension in cesarean section and poor prognosis of neonates.
Appl Bionics Biomech. 2022;2022:2468114.
|
| 7 |
Wang HZ, Chen HW, Fan YT, Jing YL, Song XR, She YJ.
Relationship between body mass index and spread of spinal anesthsia in pregnant women: a randomized controlled trial.
Med Sci Monit. 2018;24:6144-6150.
|
| 8 |
Benevides ML, Borges AKC, Seror LFG, et al.
Body mass index and clinical outcomes during cesarean section under spinal anesthesia.
Int J Anesth Clin Med. 2022;10(2):44-51.
|
| 9 |
van Dyk D, Dyer RA, Bishop DG.
Spinal hypotension in obstetrics: context-sensitive prevention and management.
Best Pract Res Clin Anaesthesiol. 2022;36(1):69-82.
|
| 10 |
Kinsella SM, Carvalho B, Dyer RA, et al.
International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia.
Anaesthesia. 2018;73(1):71-92.
|
| 11 |
George RB, McKeen DM, Dominguez JE, Allen TK, Doyle PA, Habib AS.
A randomized trial of phenylephrine infusion versus bolus dosing for nausea and vomiting during cesarean delivery in obese women.
Can J Anaesth. 2018;65(3):254-262.
|
| 12 |
Nani FS, Torres MLA.
Correlation between the body mass index (BMI) of pregnant women and the development of hypotension after spinal anesthesia for cesarean section.
Rev Bras Anestesiol. 2011;61(1):21-30.
|
| 13 |
Yang C, Meng Q, Cheng Y, Huang S, Yu X.
Effect of maternal body mass index on the prophylactic dose of phenylephrine for preventing hypotension in parturients after spinal anaesthesia.
Anaesth Crit Care Pain Med. 2022;41(2):101035.
|
| 14 |
Lee Y, Balki M, Parkes R, Carvalho JCA.
Dose requirement of intrathecal bupivacaine for cesarean delivery is similar in obese and normal weight women.
Rev Bras Anestesiol. 2009;59(6):674-683.
|
| 15 |
Allen TK, George RB, White WD, Muir HA, Habib AS.
A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery.
Anesth Analg. 2010;111(5):1221-1229.
|
| 16 |
Ortiz-Gómez JR, Palacio-Abizanda FJ, Morillas-Ramirez F, Fornet-Ruiz I, Lorenzo-Jiménez A, Bermejo-Albares ML.
Reducing by 50% the incidence of maternal hypotension during elective caesarean delivery under spinal anesthesia: Effect of prophylactic ondansetron and/or continuous infusion of phenylephrine—a double-blind, randomized, placebo controlled trial.
Saudi J Anaesth. 2017;11(4):408-414.
|
| 17 |
Xiao F, Shen B, Xu WP, Feng Y, Ngan Kee WD, Chen XZ.
Dose-response study of 4 weight-based phenylephrine infusion regimens for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia.
Anesth Analg. 2020;130(1):187-193.
|
| 18 |
Buthelezi AS, Bishop DG, Rodseth RN, Dyer RA.
Prophylactic phenylephrine and fluid co-administration to reduce spinal hypotension during elective caesarean section in a resource-limited setting: a prospective alternating intervention study.
Anaesthesia. 2020;75(4):487-492.
|
| 19 |
Nikooseresht M, Seifrabiei MA, Hajian P, Khamooshi S.
A clinical trial on the effects of different regimens of phenylephrine on maternal hemodynamic after spinal anesthesia for cesarean section.
Anesth Pain Med. 2020;10(4):e58048.
|
| 20 |
Hirose N, Kondo Y, Maeda T, Matsui M, Matsuda M, Suzuki T.
Prophylactic infusion of phenylephrine is effective in attenuating the decrease in regional cerebral blood volume and oxygenation during spinal anesthesia for cesarean section.
Int J Obstet Anesth. 2019;37:36-44.
|
| 21 |
Fletcher J, Cockerham R.
Spinal-induced hypotension at caesarean section.
Anaesth Intensive Care Med. 2022;23(6):328-330.
|
| 22 |
Kuhn JC, Hauge TH, Rosseland LA, Dahl V, Langesæter E.
Hemodynamics of phenylephrine infusion versus lower extremity compression during spinal anesthesia for cesarean delivery: a randomized, double-blind, placebo-controlled study.
Anesth Analg. 2016;122(4):1120-1129.
|
| 23 |
Singh PM, Singh NP, Reschke M, Ngan Kee WD, Palanisamy A, Monks DT.
Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for Caesarean delivery: a Bayesian network meta-analysis of fetal and maternal outcomes.
Br J Anaesth. 2020;124(3):e95-e107.
|
| 24 |
Tan HS, Habib AS.
The optimum management of nausea and vomiting during and after cesarean delivery.
Best Pract Res Clin Anaesthesiol. 2020;34(4):735-747.
|
| 25 |
Heesen M, Kölhr S, Rossaint R, Straube S.
Prophylactic phenylephrine for caesarean section under spinal anaesthesia: systematic review and meta-analysis.
Anaesthesia. 2014;69(2):143-165.
|
| 26 |
Shafeinia A, Ghaed MA, Nikoubakht N.
The effect of phenylephrine infusion on maternal hemodynamic changes during spinal anesthesia for cesarean delivery.
Anesth Pain Med. 2020;10(1):e99094.
|
| 27 |
Ngan Kee WD, Khaw KS, Ng FF.
Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section.
Br J Anaesth. 2004;92(4):469-474.
|
| 28 |
Dennis AT, Lamb KE, Story D, et al.
Associations between maternal size and health outcomes for women undergoing caesarean section: a multicentre prospective observational study (The MUM SIZE Study).
BMJ Open. 2017;7(6):e015630.
|