Abstract
Background
Sedation/analgesia (S/A) is widely used to relieve patients’ anxiety and discomfort during colonoscopy. Their effects on abdominal pain after colonoscopy have never been fully investigated.
Methods
The prospective study consecutively recruited 494 healthy patients having a screen colonoscopy examination as part of their health checkup. They were divided into two groups based on individuals’ decision to receive sedation and analgesia or not. In the S/A group, 374 patients received midazolam and alfentanil during colonoscopy with standard monitoring , while in the non-sedation/analgesia (NSA) group, 120 patients received no analgesics. Severity and duration of abdominal pain (ordinal scale: 1 = none, 4 = severe) were assessed at the end of stay in the postanesthetic care unit (the S/A group) or examination room (the NSA group) and before discharge. Side effects S/A during (hypotension, hypoxemia) and after (nausea, vomiting, dizziness) colonoscopy were also recorded. All patients were closely monitored during the whole course of the health check-up package.
Results
The mean doses of midazolam and alfentanil were 4.06 mg and 813.12 μg, respectively. Patients in the NSA group had two-fold risks of moderate to severe abdominal pain compared to those in the S/A group (16.67% vs. 9.36%, odds ratio = 1.937, 95% confidence interval: 1.010–3.625). The NSA group also had a higher incidence of abdominal pain 120 minutes after examination (33.33% vs. 16.04%,
Conclusion
Optimal S/A with midazolam and alfentanil decreases the severity and duration of postcolonoscopic abdominal pain.
Keywords
abdominal pain, colonoscope, sedation/analgesia
Introduction
Colonoscopy is commonly used as a diagnostic and screening method for colorectal neoplasms. However, gas insufflation and intraluminal instrumentation during colonoscopy cause colonic distension and abdominal pain. The abdominal pain may persist for more than 24 hours 1 and even lead to admission 2 and unplanned absence from work. 3 Its incidence ranges from 5.3% to 45.0% in different studies, depending on their design, definition of abdominal discomfort, and which time patients were asked. It may also decrease patients’ satisfaction and their willingness to accept another colonoscopy if it is necessary in the future.
Several methods, including using CO 2 instead of air for insufflation 4 or total colonic decompression after colonoscopy 5 , were tried to decrease abdominal discomfort with different degrees of success. On the other hand, although sedation and analgesia have been widely used to decrease patients’ anxiety and discomfort during colonoscopy, their effect on abdominal pain after colonoscopy, including severity, incidence, and duration, has not been clarified yet. In the current study, we test the hypothesis that among patients undergoing colonoscopy, those who received optimal sedation/analgesia (S/A) will suffer from less severe and shorter-lived postcolonoscopic abdominal pain.
Methods
Study Design and Population
After obtaining approval from the Ethics Committee of our hospital and written informed consent, asymptomatic healthy patients who received total colonoscopic examination as part of their self-paid medical health check-up were recruited consecutively in this prospective study. They were all provided with the information about S/A during colonoscopy, including its benefits and possible adverse effects. Then, they decided whether to receive S/A or not by themselves. Exclusion criteria include irritable bowel syndrome by Rome II criteria, known colorectal pathologies, and other potential causes of abdominal pain before colonoscopy. Patients were further excluded if any therapeutic procedure, for example, polypectomy, was performed during examination. Patients were divided into two groups: S/A group, in which patients received S/A; non-sedation/analgesia (NSA) group, in which patients refused to have S/A. Their demographic characteristics and medical histories were reviewed by physicians not involved in this study.
S/A and Colonoscopy
In the examination suite, monitors were provided to patients of the S/A group with continuous electrocardiogram, periodic noninvasive blood pressure measurement, and pulse oxymetry. After obtaining a baseline hemodynamic profile, patients were put in the lateral decubitus position for colonoscopy. For patients receiving S/A, supplemental oxygen 3 L per minute via nasal cannula was given after entering the examination suite. They were also encouraged to take deep breaths as preoxygenation. Induction of S/A with intravenous midazolam 0.05 mg/kg and alfentanil 10 μg/kg was given by staff anesthesiologists. After confirming patients’ loss of consciousness, endoscopists performed rectal digital examination first, then colonoscopy. During examination, a supplemental bolus of midazolam 0.5 mg with alfentanil 100 μg was given whenever the patient expressed nocifensive behavior such as withdrawal, facial grimacing, or verbal complaints about discomfort. Hypoxemia (SpO 2 < 90%) or hypotension (systolic blood pressure drops > 25% of baseline value) were treated with an oxygen face mask (6 L/min) or mask ventilation, and a bolus of intravenous ephedrine (8 mg), respectively. Major complications were defined as severe cardiopulmonary compromise for which endotracheal intubation or continuous inotropic support were necessary, unplanned admission, or mortality.
After examination, patients receiving S/A stayed in the postanesthetic care unit until, and they were fully awake, had stable vital signs without oxygen or other medication, and were able to ambulate without help.
The colonoscopy was performed in the morning by one of the four experienced endoscopists. Each of them had performed at least 1,500 colonoscopies before this study (Lee YC, Chiu HM, Huang SP, Lai YP). The one-man method was used to insert the endoscope, shorten the gut, and minimize loop formation. Air was used for insufflation. All patients had the same colon preparation regimen prior to examination.
Assessment of Abdominal Pain
One trained nurse who wasn’t involved in colonoscopic examination assessed the severity of abdominal pain in the patients of the NSA group at the end of their stay in the examination room, and in the patients of the S/A group at the end of their stay in the postanesthetic care unit. The degree of abdominal pain was graded on a 4-point ordinal scale: none, mild, moderate, or severe. Before discharge, usually in the afternoon after patients finished a battery of tests, patients were evaluated again for overall severity and duration of postcolonoscopic abdominal pain, and S/A-associated side effects, including nausea, vomiting, and dizziness. All subjects were closely monitored during this period (about 4 to 6 hours). The onset and duration of postcolonoscopic abdominal pain were recorded. In addition to the duration reported by individual patients before discharge, the duration between leaving the examination room and being able to report abdominal pain for the first time at postanesthesia care unit was added to calculate the total duration of abdominal pain in group S/A.
Statistical Analysis
Quantitative data were summarized as mean
±
standard deviation (SD) and categorical variables as percentages and absolute count. Chi-square test for categorical variable, Student
Results
Patients Analysis
A total of 494 patients were recruited in this prospective study, 120 in the NSA group and 374 in the S/A group. Their demographic characteristics are listed in Table 1. There was no statistically significant difference between the two groups with respect to age and body mass index. The male/female ratio was higher in the NSA group than in the S/A group. In other words, fewer male patients chose to receive S/A than female patients (71% vs. 82%,
S/A
Among patients receiving S/A, mean total doses (induction and bolus doses) of midazolam and alfentanil were 4.06
±
0.70 mg (0.067
±
0.015 mg/kg) and 813.12
±
139.90 μg (13.31
±
3.04 μg/kg), respectively. Hypoxemia occurred in 11 patients (2.9%), and hypotension occurred in 21 patients (5.6%). The incidence of nausea and vomiting wasn’t significantly different between the NSA and S/A groups. More patients in the S/A group felt dizzy or sleepy than in the NSA group (14.7% vs. 3.3%,
|
Characteristic |
NSA group (n = 120) |
S/A group (n = 374) |
|
|---|---|---|---|
|
Age |
51.74 ± 11.25 |
51.96 ± 12.70 |
|
|
Gender (M : F) |
85 : 35 (= 2.4 : 1) |
211 : 163 (= 1.3 : 1) |
0.0054 |
|
Body mass index |
24.07 ± 3.79 |
23.38 ± 3.59 |
Abbreviations: NSA, non-sedation/analgesia; S/A, sedation/analgesia.
Abdominal Pain
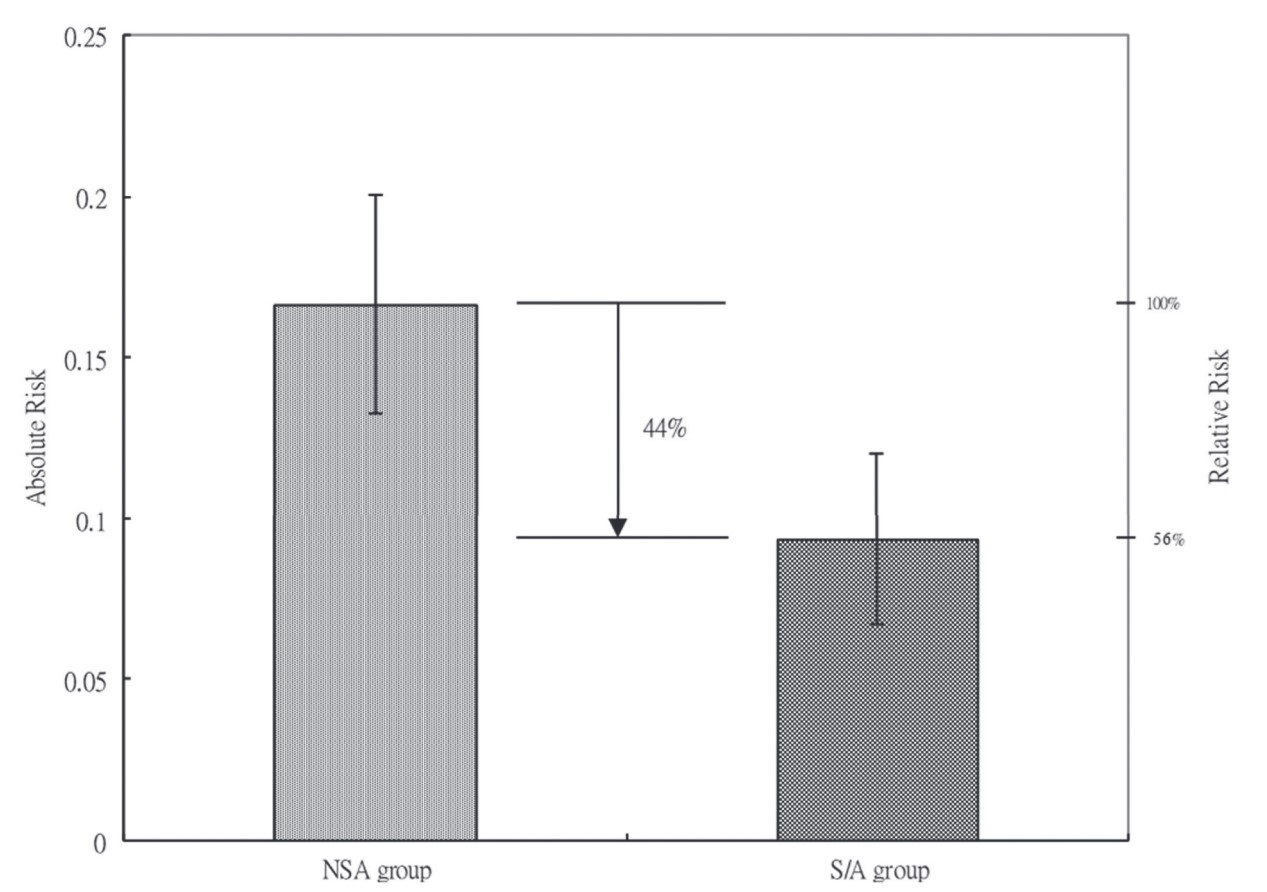
During their health checkup course after colonoscopy and before discharge, 16.67% of patients in the NSA group and 9.36% of patients in the S/A group experienced moderate to severe abdominal pain (
|
NSA group (n = 120) |
S/A group (n = 374) |
|
|
|---|---|---|---|
|
S/A-related |
|||
|
Nausea (none/mild/moderate/severe), n (%) |
118/1/0/1 (1.7) |
373/1/0/0 (0.3) |
|
|
Vomiting (none/mild/moderate/severe), n (%) |
118/1/0/1 (1.7) |
374/0/0/0 (0.0) |
|
|
Dizziness/sleepy (none/mild/moderate/severe), n (%) |
116/4/0/0 (3.3) |
319/46/8/1 (14.7) |
0.0008 |
|
Hypoxemia (SpO 2 < 90%), n (%) |
11 (2.9) |
||
|
Hypotension (SBP drops > 25% of baseline value), n (%) |
21 (5.6) |
||
|
Overall abdominal pain |
|||
|
none/mild/moderate/severe |
60/40/18/2 |
210/129/35/0 |
|
|
Total, n (%) |
60 (50.00) |
164 (43.85) |
0.24 |
|
Moderate and severe, n (%) |
20 (16.67) |
35 (9.36) |
0.026 |
|
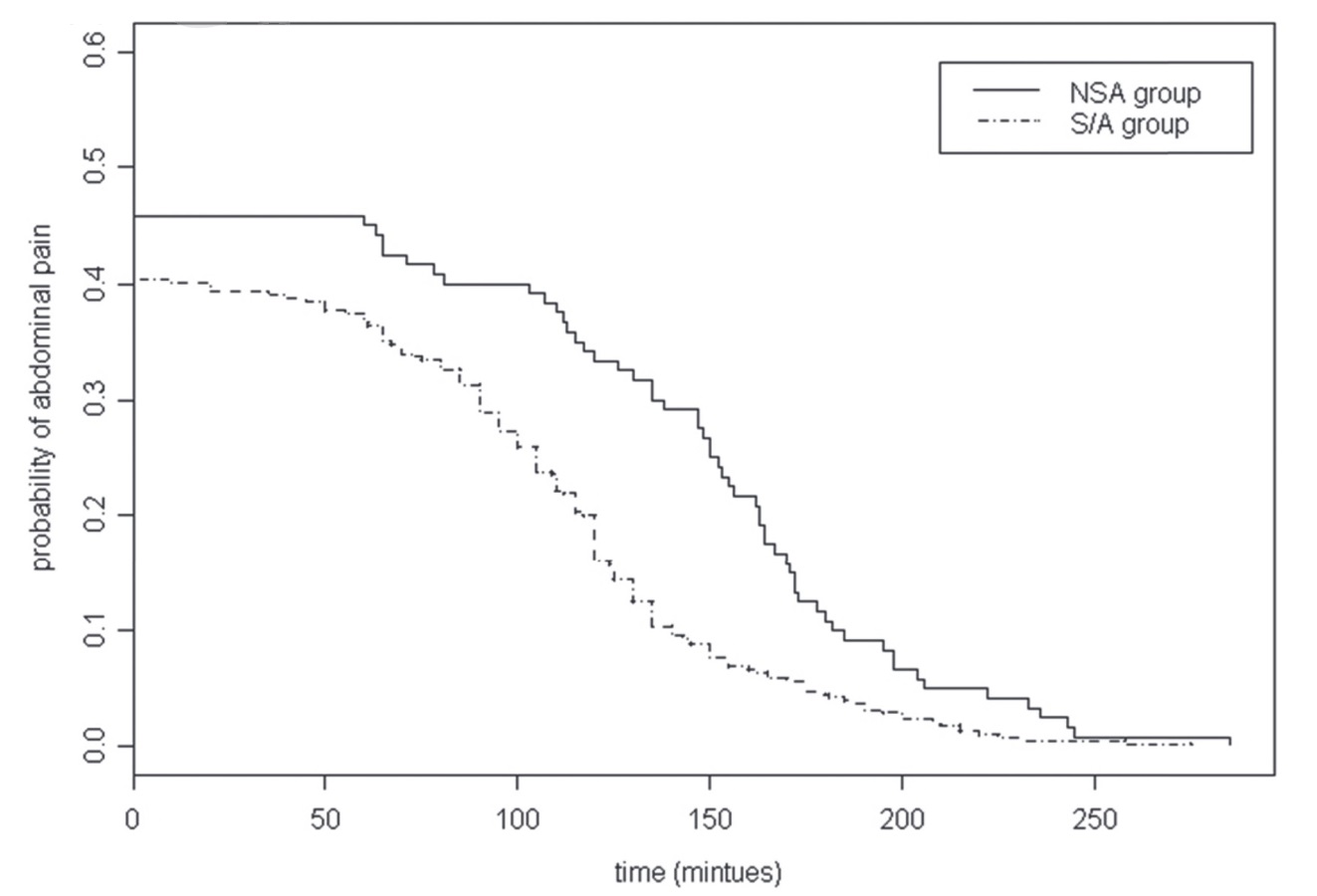
Duration (minutes) a |
153.3 ± 49.9 |
118.7 ± 47.5 |
< 0.001 |
|
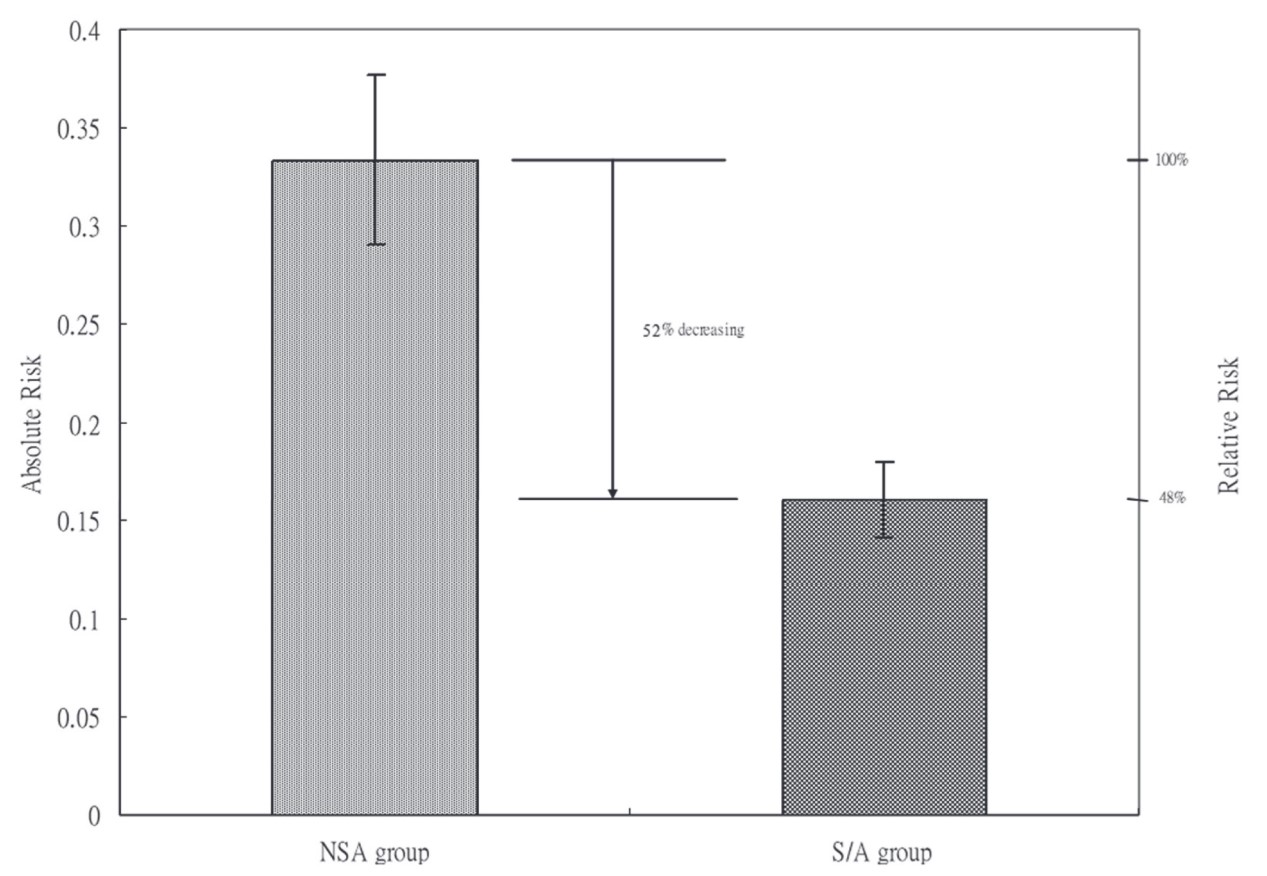
Abdominal pain at 120 minutes, n (%) |
40 (33.33) |
60 (16.04) |
< 0.001 |
|
Major complication |
0 |
0 |
Abbreviations: NSA, non-sedation/analgesia; S/A, sedation/analgesia; SBP, systolic blood pressure.
Download full-size image
Abbreviations: NSA, non-sedation/analgesia; S/A, sedation/analgesia.
Download full-size image
Abbreviations: NSA, non-sedation/analgesia; S/A, sedation/analgesia.
Download full-size image
Abbreviations: NSA, non-sedation/analgesia; S/A, sedation/analgesia.
Discussion
To date, this is the first study demonstrating that with optimal sedation and analgesia, patients will experience less discomfort not only during colonoscopy but also after examination. We’ve shown that S/A caused a 44% decrease in the incidence of moderate to severe abdominal pain, which might affect patients’ willingness to return for subsequent examination more likely. 6 Besides incidence, patients who did have abdominal pain had a more rapid recovery with S/A than those who refused it.
Although colonoscopy is a relatively non-traumatic procedure, it causes noxious stimulation by instrumentation and colorectal distension. Colorectal distension has been proved to be a noxious visceral stimulus and used as a model of visceral pain. 7,8 In animal studies, adrenoreceptor, 5-HT, GABA, NMDA, and opioid receptors located in the spinal cord are found to be involved in the processing of visceral pain caused by colorectal distension. 9-12 The mechanisms of anti-visceral pain behind the combination of midazolam and alfentanil have been proven by numerous animal studies that GABA agonists would increase the antinociceptive effect of opioids in the colorectal distension model 10 . The synergism between two distinct pharmacodynamic medicines, i.e., midazolam and alfentanil, could thus prolong the duration of action and thereby reduce the incidence and duration of postcolonoscopic abdominal pain. Figure 2 shows that, instead of 2 parallel lines, the recovery curve of the S/A group has a steeper slope than that of the NSA group. The greatest difference lies about 120 minutes after colonoscopy, far beyond the duration of action of midazolam and alfentanil. It may be explained by the prevention of visceral hyperalgesia. Visceral hyperalgesia, meaning that previous exposure to colorectal distension or irritant made subjects more sensitive and produced more severe responses to subsequent colorectal distension, was found in the rat model. 13-15 Midazolam and alfentanil, given before gas insufflation, may prevent or decrease the occurrence of visceral hyperalgesia. In humans, gas insufflation during colonoscopy is a unique clinical scenario most similar to the colorectal distension model. The clear cause-effect relationship can be established not only between gas insufflation and abdominal pain but also between treatment and pain relief. In this study, colonoscopy is a part of a health checkup program, not a single exam. Our patients did not need to stay in the hospital much longer than patients having a common outpaient colonoscopy. Thus, time spent on close follow-up and direct contact via phone communication will be markedly reduced.
In addition to gas insufflation, instrumentation and manipulation during colonoscopy may also be possible causes of postcolonoscopic abdominal pain. Thinner and more flexible endoscopes were found to be associated with less discomfort. 16 Another study showed that most pain episodes during colonoscopy coincided with the looping or straightening of the colonoscope shaft. 17 The question of whether more looping is associated with more postcolonoscopic abdominal pain still needs to be clarified.
Shah et al.
17
also found that looping was more frequent and less well tolerated in women than in men. In our study, more women chose S/A than men. The unequal gender distribution meant women might be more anxious about possible discomfort and might express a higher degree of abdominal pain. Therefore, the real benefit of S/A may be more than what our study can reveal because patients in the S/A group, although with more female patients, expressed less abdominal pain. We also have performed logistic regression analysis to control for the possible effect of gender difference. After controlling for gender, the odds ratio for the overall incidence of moderate to severe abdominal pain is 0.50 (the S/A group vs. the NSA group,
The incidence of complications related to S/A in our study, especially hypotension and hypoxemia, was comparable to other studies. 18-20 The consumption dose of midazolam and opioid (in equipotent dose) was also similar. We chose midazolam and alfentanil, instead of propofol, which is associated with faster recovery of cognitive function 18,19 because our patients regarded injection pain caused by propofol as a major side effect, and they had to stay in the hospital after colonoscopy for other health checkup exams. Early recovery and discharge are not a chief goal in this circumstance. Other minor complications, such as nausea, vomiting, and dizziness, didn’t cause much disturbance. Patients in the S/A group didn’t have more nausea or vomiting than in the NSA group. The short duration of action of alfentanil may explain that. Although S/A caused more dizziness, most was well tolerated (mild: 12.3%; moderate to severe: 2.4%).
Further study about postcolonoscopic abdominal pain may include ketamine or opioids with a longer duration of action. The former, as an NMDA receptor antagonist, may reduce abdominal pain because NMDA receptor antagonists could attenuate visceral hyperalgesia in animal models. 13,21 Prolonged abdominal pain and persistent colon distension 1 explain the possible role of opioids with the duration of action long enough to cover the recovery phase, not just the examination period.
In conclusion, our study demonstrates that intravenous midazolam 0.05 mg/kg and alfentanil 10 μg/kg can produce nearly half decrease in the incidence of abdominal pain after colonoscopy and shorten its duration. Building on the present investigation, our results highly recommend that combining sedation and analgesia medications—such as ultra-short-acting remifentanil and remimazolam—will serve as a better combination choice in the future study design.
References
| 1 |
Stevenson GW, Wilson JA, Wilkinson J, Norman G, Goodacre RL.
Pain following colonoscopy: elimination with carbon dioxide.
Gastrointest Endosc. 1992;38(5):564-567.
|
| 2 |
Bini EJ, Firoozi B, Choung RJ, Ali EM, Osman M, Weinshel EH.
Systematic evaluation of complications related to endoscopy in a training setting: a prospective 30-day outcomes study.
Gastrointest Endosc. 2003;57(1):8-16.
|
| 3 |
Newcomer MK, Shaw MJ, Williams DM, Jowell PS.
Unplanned work absence following outpatient colonoscopy.
J Clin Gastroenterol. 1999;29(1):76-78.
|
| 4 |
Church J, Delaney C.
Randomized, controlled trial of carbon dioxide insufflation during colonoscopy.
Dis Colon Rectum. 2003;46(3):322-326.
|
| 5 |
Lee JG, Vigil H, Leung JW.
A randomized controlled trial of total colonic decompression after colonoscopy to improve patient comfort.
Am J Gastroenterol. 2001;96(1):95-100.
|
| 6 |
Redelmeier DA, Kahneman D.
Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures.
Pain. 1996;66(1):3-8.
|
| 7 |
Ness TJ, Gebhart GF.
Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat.
Brain Res. 1988;450(1-2):153-169.
|
| 8 |
Ness TJ, Randich A, Gebhart GF.
Further behavioral evidence that colorectal distension is a “noxious” visceral stimulus in rats.
Neurosci Lett. 1991;131(1):113-116.
|
| 9 |
Song XJ, Zhao ZQ.
Involvement of NMDA and non-NMDA receptors in transmission of spinal visceral nociception in cat.
Zhongguo Yao Li Xue Bao. 1999;20(4):308-312.
|
| 10 |
Hara K, Saito Y, Kirihara Y, Yamada Y, Sakura S, Kosaka Y.
The interaction of antinociceptive effects of morphine and GABA receptor agonists within the rat spinal cord.
Anesth Analg. 1999;89(2):422-427.
|
| 11 |
Danzebrink RM, Gebhart GF.
Evidence that spinal 5-HT1, 5-HT2 and 5-HT3 receptor subtypes modulate responses to noxious colorectal distension in the rat.
Brain Res. 1991;538(1):64-75.
|
| 12 |
Danzebrink RM, Gebhart GF.
Antinociceptive effects of intrathecal adrenoceptor agonists in a rat model of visceral nociception.
J Pharmacol Exp Ther. 1990;253(2):698-705.
|
| 13 |
Coutinho SV, Urban MO, Gebhart GF.
The role of CNS NMDA receptors and nitric oxide in visceral hyperalgesia.
Eur J Pharmacol. 2001;429(1-3):319-325.
|
| 14 |
Gschossmann JM, Coutinho SV, Miller JC, et al.
Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat.
Neurogastroenterol Motil. 2001;13(3):229-236.
|
| 15 |
Friedrich AE, Gebhart GF.
Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla.
Pain. 2003;104(1-2):93-101.
|
| 16 |
Farraye FA, Horton K, Hersey H, Trnka Y, Heeren T, Provenzale D.
Screening flexible sigmoidoscopy using an upper endoscope is better tolerated by women.
Am J Gastroenterol. 2004;99(6):1074-1080.
|
| 17 |
Shah SG, Brooker JC, Thapar C, Williams CB, Saunders BP.
Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging.
Endoscopy. 2002;34(6):435-440.
|
| 18 |
Ulmer BJ, Hansen JJ, Overley CA, et al.
Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists.
Clin Gastroenterol Hepatol. 2003;1(6):425-432.
|
| 19 |
Sipe BW, Rex DK, Latinovich D, et al.
Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists.
Gastrointest Endosc. 2002;55(7):815-825.
|
| 20 |
Wexner SD, Garbus JE, Singh JJ; SAGES Colonoscopy Study Outcomes Group.
A prospective analysis of 13,580 colonoscopies.
Surg Endosc. 2001;15(3):251-261.
|
| 21 |
Gaudreau GA, Plourde V.
Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity.
Behav Brain Res. 2004;150(1-2):185-189.
|