Abstract
Background
Critical illness is associated with oxidative stress and insulin resistance. These conditions affect the clinical outcomes in intensive care unit (ICU). The aim of this study was to determine whether intervention with α-lipoic acid (ALA) influences the oxidative stress, insulin resistance, and clinical outcomes in critically ill patients.
Method
In this randomized double-blind placebo-controlled trial, 80 critically ill patients who were expected to stay at least seven days in the ICU and required enteral feeding were randomly allocated to two equal groups to receive either ALA (900 mg) or placebo daily for 10 days. Serum levels of total antioxidant capacity (TAC), malondialdehyde (MDA), insulin, glucose (GLC), C-reactive protein (CRP), albumin (Alb), prealbumin (preAlb), total protein (total-pr) and total lymphocyte count (TLC) as well as homeostasis model assessment-estimated insulin resistance (HOMA-IR) were measured at baseline and at the end of ALA supplement phase. Clinical outcomes (length of ICU/hospital stay, ICU/hospital mortality, and 28-day mortality and ventilator free days) were also recorded.
Results
TAC increased signifi cantly in the ALA supplemented group compared to the placebo group (p < 0.001). Moreover, serum levels of GLC decreased signifi cantly in the ALA group compared to lack of changes in the placebo group (p = 0.011). ALA supplementation also hindered an increase in HOMA-IR (p = 0.015). There were no signifi cant differences in other biochemical markers and clinical outcomes between the two groups.
Conclusion
ALA may be an effective supplement to improve antioxidant defense and insulin resistance in critically ill patients.
Keywords
α-lipoic acid; antioxidant; critical illness; insulin resistance; oxidative stress;
1.Introduction
Critical illness is associated with several metabolic, endocrine and biochemical changes.1 The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) increases in critically ill patients.2 Several studies indicated low levels of antioxidant such as ascorbic acid, carotenoids, vitamin A, vitamin E, selenium and glutathione in critically ill patients.3-6 Decreased activities of enzymatic antioxidant defense systems including superoxide dismutase (SOD), catalase, and glutathione peroxidase were reported as well.7,8
Oxidative stress is the consequence of the imbalance between enzymatic and non-enzymatic defense systems and free radicals.2 It is a contributing factor to intensive care unit (ICU) patient complications such as acute respiratory distress syndrome, multiple organ dysfunction, and systemic inflammatory response syndrome.2,9 Free radicals are liable for tissue injury by damaging lipid, protein and DNA.2 Furthermore, pro-inflammatory cytokines transcription is activated by free radicals, oxidative stress and virus or bacterial infection in critically ill patients. This cascade stimulates inflammatory response.10 Stress induced hyperglycemia is another common problem in ICU patients. Increasing counter regulatory hormones (i.e. glucagon and catecholamines) and high circulatory level of cytokines (i.e. interleukin- 6 [IL-6] and tumor necrosis factor-α [TNF-α]) are the causes of stress induced hyperglycemia in critically ill patients. Excessive glucose (GLC) production as well as insulin resistance could account for this metabolic milieu.11,12 The relationship between hyperglycemia, mortality and morbidity in medical, trauma and surgical ICU patients has been well established.11,13-15 Lipoic acid (LA = C8H14O2S2) is a unique antioxidant because both reduced and oxidized forms of this supplement have antioxidant property. This amphiphilic antioxidant can directly scavenge ROS/RNS and regenerate endogenous antioxidants such as vitamin C and gluthatione.16 It also is a cofactor of mitochondrial dehydrogenase complexes and mitochondrial respiratory enzymes.16 Several studies have demonstrated that LA can stimulate GLC uptake by fat and muscle cells and thus decrease insulin resistance in prediabetes and type 2 diabetic patients.17-19 Therefore, it seems logical that supplementation with α-lipoic acid (ALA) could be a valuable approach in critically ill patients. To our knowledge no clinical study has ever evaluated the antioxidant activity of ALA on improving clinical outcomes such as length of ventilator free days, length of stay (LOS) in ICU, 28-day mortality and biochemical indices such as insulin resistance and oxidative stress markers in critically ill patients. We performed this trial to evaluate the efficacy of ALA supplementation on clinical and biochemical outcomes of critically ill patients.
2.Methods
This randomized, double-blind, placebo-controlled, two-arm parallel trial was started on May 2015 and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice recommendations. The protocol of this study was reviewed and approved by the Ethics Committee of Shiraz University of Medical Sciences, Iran (ethical approval number: ec-91-6423). Informed consent was obtained prior to the randomization of eligible patients (when possible) or their next of kin. Eligible participants were ICU patients aged 18 years or older, who were candidates for enteral feeding and expected to stay in ICU for at least seven days. Patients were excluded from the study if they were transferred from other hospitals or ICUs, couldn’t tolerate enteral feeding, were human immunodeficiency virus (HIV) or hepatitis B/C surface antigen positive, patients with severe malnutrition prior to ICU admission, patients with severe liver or kidney failure or immunosuppressive disease and patients who take part in another interventional study simultaneously.
A sample size of 40 patients per group was obtained with a mean difference of 0.25 based on the decrease in the mean LOS in ICU stay in ICUs of Shiraz hospitals, an SD of 4 and a probability of 80% at the predetermined level of α = 0.05. Out of 270 sequentially admitted patients to emergency ICU in Nemazee hospital, 80 critically ill patients prospectively participated in this trial. These patients were randomly assigned in a 1:1 ratio into two groups:
(1) The patients in the treatment group received three capsules of ALA (900 mg) manufactured by general nutrition center company (GNC, Pittsburgh, PA, USA) daily for 10 days with the start of enteral feeding in 24 to 48 first hour of ICU admission.
(2) The patients in the control group received three capsules of placebo (medical starch) which were prepared by Shiraz school of pharmacy, daily for 10 days with the start of enteral feeding in 24 to 48 first hour of ICU admission.
Blocked randomization with a fixed block size of four was performed by a nurse who had no clinical involvement in the study using Random Allocation Software.20 The placebo and ALA capsules were completely similar in size, color, smell and weight. The content of capsules was mixed with water daily by ICU patients’ nurse; the solution was connected to the administration set and was ready for immediate consumption by the patient.
Abbott Nutrition products were used as enteral feeding. Patients’ intake was recorded daily during 10 days of the study. This made it possible to calculate the mean intake of energy and nutrients during the study and compare that with nutritional prescription. Prescription of energy for patients was based on multiplying the patients’ actual (for patients with body mass index [BMI] up to 29.9) or target weight (for BMI ≥ 30) in kilogram by 25–30 kcal/kg. Computation of prescribed protein was based on severity and type of patients’ disease (0.8–1.5 g/kg/day). Fifty to sixty five percent of feeding goal was determined as cutoff point for energy intake in study patients.21
Formulas were administered by nasogastric, gastrostomy, or jejunostomy tube at the discretion of specialist critical care physician based on our regional enteral feeding guideline. The method of feeding in the ward was bolus feeding. Dietary supplements (ALA or placebo) were given to patients 2 hours after first morning feeding time, because food intakes reduce the bioavailability of ALA and the recommendation is taking up ALA 1 hour before or two hours after eating.16,22 The ALA dosage and duration of the study were chosen on the basis of literature review.16,23-26
Insulin sliding scale protocol was used in emergency ICU. Regular insulin usage was recorded for each patient during 10 days of study.
Before the onset and after the end of supplementation phase (11th day), 7 cc venous blood samples were drawn out from each patient. Blood samples were centrifuged at 2,000 g/min for 10 min; the serum was then separated, frozen immediately and stored at -70 °C until analysis. Serum levels of total antioxidant capacity (TAC) (mM), malondialdehyde (MDA) (μmol/L), IL-6 (pg/mL), C-reactive protein (CRP) (mg/dL), GLC (mg/dL), Insulin (μIU/mL), albumin (Alb) (g/dL), prealbumin (preAlb) (mg/dL), total protein (total-pr) (g/dL) and total lymphocyte count (TLC) were measured in all patients at baseline and at eleventh day of study. Insulin resistance was also measured by homeostasis model assessment-estimated insulin resistance (HOMA-IR) based on this equation: [GLC (mmol/L) × insulin (μIU/mL)] / 22.5.27
Serum concentrations of MDA were measured by the modified thiobarbituric acid method (spectrophotometric method).28 The serum level of TAC was measured by colorimetric assay (cayman, Ann Arbor, MI, USA). Serum levels of IL-6 and insulin were determined using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, Santiago, CA, USA for IL-6 and DRG, Marburg, Germany for Insulin). The auto-analyzer was used to measure the GLC, total-pr and Alb levels. PreAlb level was also assessed using turbidometric method (Biosystems, Barcelona, Spain). Nephelometric assay was used to measure CRP levels in serum (Binding Site Group Ltd., Birmingham, UK).
Clinical events including, diarrhea occurrence during ICU stay, LOS in ICU, LOS in hospital, number of ventilator free days, 28-day mortality and mortality rate during ICU or hospital stay were also recorded.
Statistical analysis was done using SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean ± SD or median (interquartile range [IQR]) based on normal/abnormal distribution. Normally distributed data were compared between the two groups by an independent sample t-test; skewed data were compared by the Mann–Whitney U-test and categorical data such as sex by χ2 test. Pair t-test was used to compare the means in each group of study. p values ≤ 0.05 were considered statistically significant. The primary endpoint of this study was evaluating the LOS in ICU in an intention-to-treatment population. This trial was registered with ClinicalTrial.gov, NO. NCT01888861.
3.Results
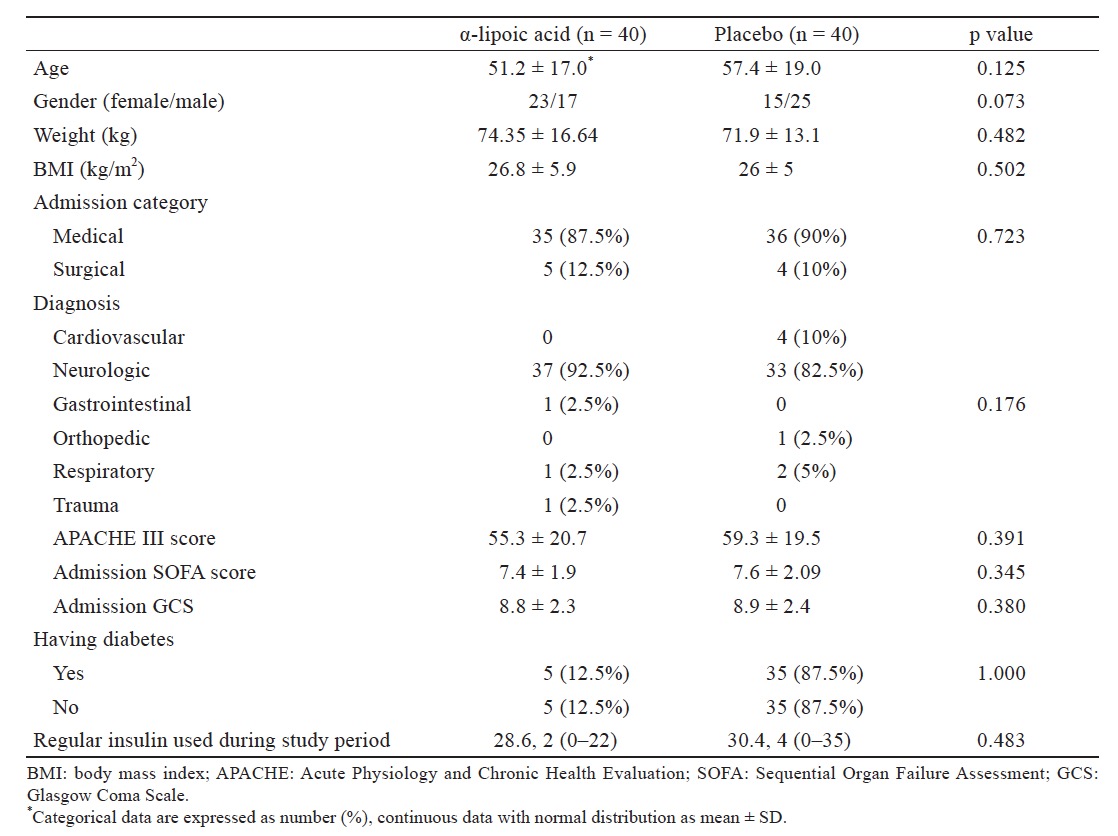
Out of 80 patients involved in this study, 1 patient in the treatment group and 2 in the placebo group were excluded from the study due to death before completing the study period (Fig. 1 ). Baseline characteristics of patients are shown in Table 1 ; no statistically significant differences were observed between these groups in terms of age, gender, weight, BMI, admission category, Glasgow Coma Scale (GCS), Acute Physiology and Chronic Health Evaluation III (APACHE III) score and Sequential Organ Failure Assessment (SOFA) score. This table also implies that there isn’t any significant difference in amount of insulin used between two groups during study. Accidentally, the patients in this single center study were predominantly medical patients with neurologic reason of admission.
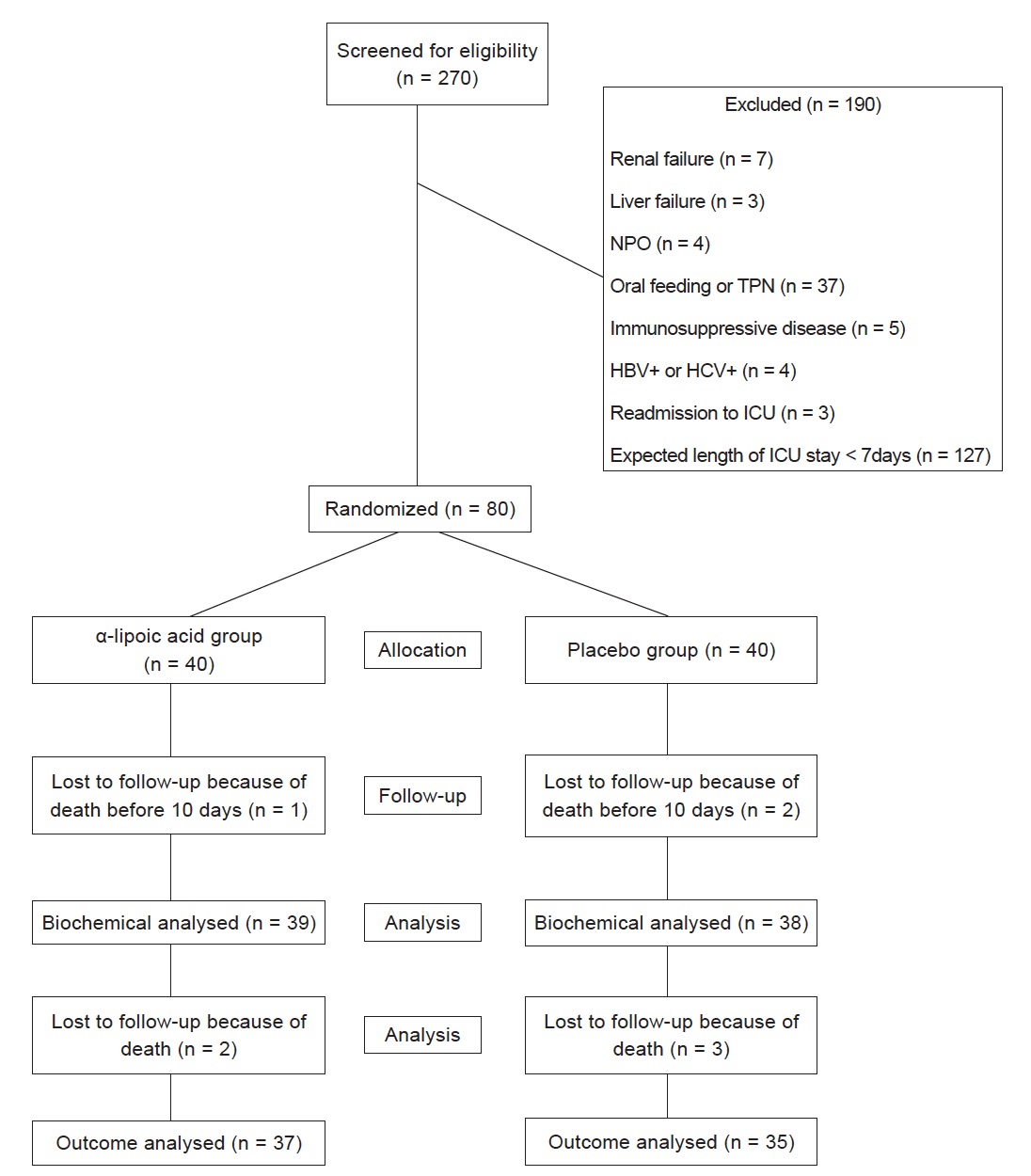
Download full-size image
NPO: nothing by mouth; TPN: total parenteral nutrition; HBV: hepatitis B virus; HCV: hepatitis C virus; ICU: intensive care unit.
Download full-size image
Biochemical measured parameters before and after treatment phase (10 days) of the study are presented in Table 2 . TAC concentration increased significantly in the ALA group (p = 0.007) while this oxidative-stress marker significantly decreased in the placebo group (p = 0.005); when changes in this marker was compared between the two groups, increment in TAC in the ALA group was significantly different from the corresponding decrement in the placebo group (p < 0.001). Likewise, a decrement in the serum level of GLC in the ALA group was significantly different from lack of changes in the placebo group. (p = 0.011) Moreover, insulin resistance (HOMA- index) increased in both groups; even so, this increment was significantly lower in the ALA group (p = 0.015). Changes in other biochemical parameters were not significantly different between the two groups.

Download full-size image
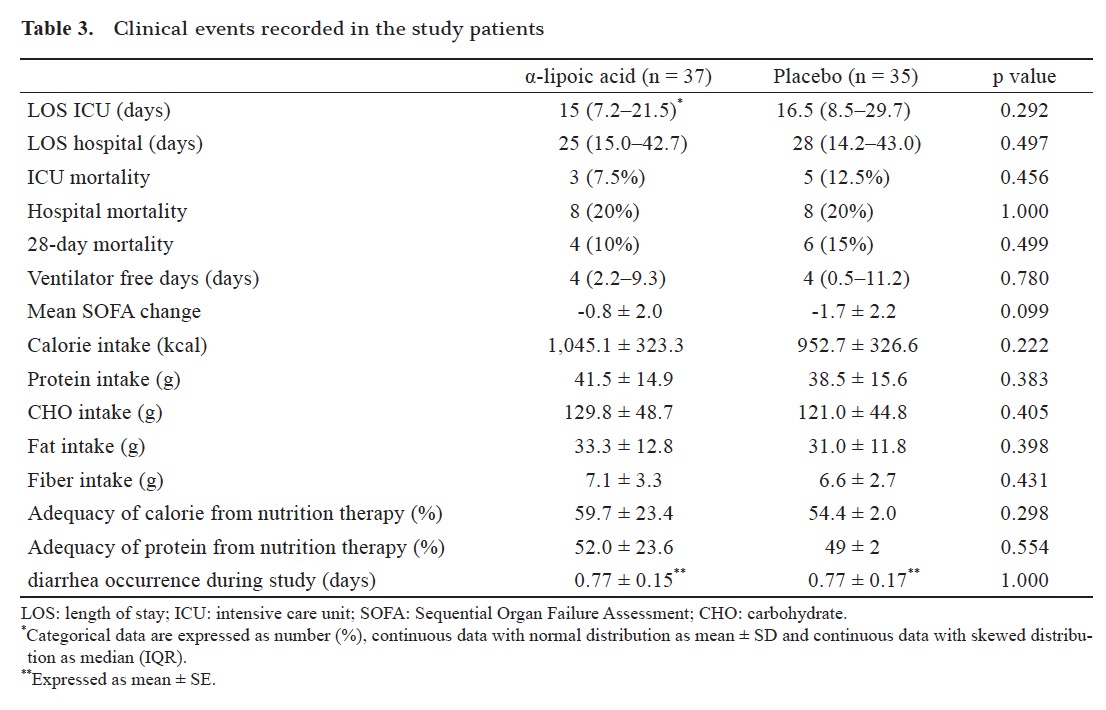
Clinical outcomes of enrolled patients after randomization and completing the study are displayed in Table 3 . The difference between the LOS in ICU and the LOS in hospital stay was not significant between the two groups of the study. However, LOS in ICU was lower in the ALA group compared to the placebo group (the averages were 16.5 and 22.8 days respectively). ALA supplementation also didn’t show a significant effect on mortality rate during ICU and hospital stay or on 28 day mortality (Table 3 ). There were no significant differences in the number of ventilator- free days, SOFA changes and diarrhea occurrence during ICU stay between the two groups, too (Table 3 ). Other adverse events did not occur in patients during intervention.
Table 3 also shows that the means of calorie and macronutrient including carbohydrate, protein, and fat and fiber consumption were similar between groups during the ten days of the study. The adequacy of calorie and protein from nutrition therapy were similar between ALA and placebo groups too (Table 3 ).
Download full-size image
4.Discussion
The efficacy of ALA on oxidative stress, insulin resistance, biochemical and nutritional indices along with clinical outcomes such as ICU/hospital LOS, mortality and ventilator independency in ICU patients were evaluated in this study. The results indicate that TAC increased significantly in the ALA supplemented group compared to the placebo group. Moreover, serum levels of GLC decreased significantly in the ALA group compared to lack of changes in the placebo group. ALA supplementation also hindered an increase in insulin resistance significantly.
The role of oxidative stress in exacerbation of the organ injury is established by systemic inflammatory response syndrome, ischemic reperfusion injury and stress induced hyperglycemia in critically ill patients. 13,26 The high oxidative stress in these patients is the consequence of imbalance between antioxidant defense system and oxidizing species. Previous studies demonstrated low serum concentration of micronutrients containing antioxidants6,29-31 and reduced enzymatic system activity in detoxification of free radicals.7,31,32 Abilés et al. showed that ICU stay is associated with decreased TAC if patients didn’t take adequate amount of antioxidants.32 Evidence confirmed this biochemical state in ischemic and hemorrhagic stroke, cerebrovascular disease and other neurologic problems.33-35 A number of studies showed that low endogenous stores of antioxidants are associated with more free radical generation, an increase in systemic inflammatory response, succeeding cell injury, increased morbidity, and higher mortality in the critically ill patients.6,36 Oxidative stress is a possible contributor towards pathophysiological consequences of stroke too.33 Therefore, provision of antioxidants for neurologic and critically ill patients may alleviate the complications.
In the present study, a significant increase in the serum TAC level in the ALA group compared to the placebo group was observed. Serum TAC indicates serum lipid- and aqueous-soluble antioxidants including vitamins, lipids, glutathione, uric acid, proteins, etc. So toward serum LA changes as a biological antioxidant that derives from octanoic acid and cysteine, serum TAC affected. However increment of reduced form of vitamin E, vitamin C and glutathione by ALA can also affect TAC level.16 Animal studies indicated that ALA can lessen oxidative lung injury by decreasing nuclear factor kappa B (NF-ĸB) activation in the lung tissues and increasing the antioxidant capacity of the lungs in sepsis and septic shock.37,38 In an interventional study by Skibska et al., ALA increased the antioxidant capacity of the plasma in lipopolysaccharide- shocked animals.39 However, intravenous administration of one dose antioxidant (N-acetylcysteine, ascorbic acid and α-tocopherol) didn’t affect the serum TAC in patients with septic shock in Galley et al.’s study.40 LA and its reduced form dihydrolipoic acid (DHLA) are potent antioxidants by a synergistical action.41,42 They both quench oxidative stress through scavenging free radicals, regenerating endogenous antioxidants and chelating redox-active metals. 41 This study also showed that ALA resulted in a significant reduction in blood GLC level and prevention of the increase in insulin resistance (HOMA-index) in critically ill patients. ALA has the ability for glycemic control by decreasing insulin resistance. It has been demonstrated by Ansar et al.43 and Kamenova et al.44 through decreasing blood GLC, insulin resistance and improving insulin sensitivity in type 2 diabetic patients. Vidović et al. also observed that ALA supplementation decreased GLC level in patients with schizophrenia.45 In critically ill patients, insulin resistance is the consequence of increased counter-regulatory hormones and cytokins, induced by oxidative stress and its signaling pathway.11 Stress sensitive signaling pathway is activated by free radicals (i.e. ROS) as signaling molecules and increases the transcription of proinflammatory cytokines and the production of sorbitol and advanced glycation end product which have a key role in causing insulin resistance.46 LA and DHLA affect stress sensitive signaling pathway through neutralizing ROS and thus decreasing activation of NF-ĸB, c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) , p38 mitogen-activated protein kinase (MAPK), and hexosamine as mediators of pathway.41,46 ALA can also stimulate both glucose transporter type 4 (GLUT4) translocation from golgi to the plasma membrane and its activation in muscle cells to increase GLC transport.47 Furthermore, ALA can decrease insulin resistance by protecting insulin receptor from oxidative damage and maintaining its integrity.46 Up to now, insulin therapy was the treatment for stress induced hyperglycemia in critically ill patients.48 However, complications such as hypoglycemia are assumed to happen by insulin infusion.49,50 As Arabi et al. showed, intensive insulin therapy in critically ill patients is associated with a significant increase in hypoglycemia incidence in these patients.51 A meta-analysis conducted by Griesdale et al. concludes that intensive insulin therapy increased the risk of hypoglycemia significantly and had no general mortality benefit among critically ill patients.52 Although our study demonstrates that ALA can decrease the blood GLC without hypoglycemic effect, it didn’t show a significant effect on clinical outcomes including mortality, hospital or ICU LOS, ventilator free days and SOFA score. Surveys such as that conducted by Heyland et al. have shown that antioxidants (selenium, β-caroten, vitamin C, vitamin E and zinc) have no effect on 28-day mortality, hospital mortality and number of organ failure.53 However, in 2012, Manzanares et al. published a meta-analysis, in which they concluded that combined antioxidants were associated with a significant reduction in duration of mechanical ventilation and a significant reduction in mortality among patients with higher risk of death.54 Different confounders may have roles in our clinical results. Manzanares et al.’s meta-analysis showed a significant effect of antioxidant supplementation on clinical outcomes in randomized clinical trials with mortality higher than 10% in control group.54 Critically ill patients with more severe condition and higher mitochondrial dysfunction showed more improvement with antioxidant supplementation.54 The levels of energy and protein intake were maybe other confounders.
The main limitations of the present study were short duration and mixed population of the study, further clinical trial with longer duration, and different doses of ALA; actually, a specific target group of ICU patients is needed. In addition, evaluating ALA adjunct to conventional insulin therapy or other antioxidants is also recommended to confirm its safety and efficacy in long term use. In present study relatively low mortality rate (7.5%) in ALA group compare to the control (12.5%) despite of the same Appache score levels may show mortality benefit of ALA if used in the setting of high mortality ICU patients but a specific clinical trial is recommended. In summary, this study showed the efficacy of ALA in decreasing blood GLC, preventing the increase of insulin resistance and improving TAC in critically ill patients. We could conclude that ALA may be a suitable adjunct therapy to control intermediate hyperglycemia and improve oxidative stress in ICU patients.
5.Acknowledgements
The authors would like to thank the cooperation of Nemazee hospital staff. The authors would also like to thank Dr. Nasrin Shokrpour at RCC for editorial assistance.
6.Authorship
Najmeh Hejazi participated in gathering, analysis and interpreting the data, carrying it out and writing the article. Dr. Zohreh mazloom, Dr. Farid Zand and Dr. Reza Nikandish had roles in designing the study, revising the article critically and approving the article. Dr. Abbas Rezaeian Zadeh participates in designing the study and analysis and interpreting the data.
7.Funding
This study was extracted from Ph.D thesis written by Najmeh Hejazi and was financially supported by Shiraz University of Medical Sciences grant No. 91-6423.
8.Conflicts of Interest
None declared.
References
| 1 |
Verbruggen SC, Joosten KF, Castillo L, van Goudoever JB.
Insulin therapy in the pediatric intensive care unit.
Clinical Nutr 2007;26:677–690.
|
| 2 |
Goodyear-Bruch C, Pierce JD.
Oxidative stress in critically ill patients.
Am J Criti Care 2002;11:543–551.
|
| 3 |
Schorah CJ, Downing C, Piripitsi A, et al.
Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients.
Am J Clin Nutr 1996;63:760–765.
|
| 4 |
Curran F, Sattar N, Talwar D, Baxter JN, Imrie CW.
Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis.
Br J Surg 2000;87:301–305.
|
| 5 |
Hammarqvist F, Luo JL, Cotgreave IA, Andersson K, Wernerman J.
Skeletal muscle glutathione is depleted in critically ill patients.
Crit Care Med 1997;25:78–84.
|
| 6 |
Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR.
Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction.
Crit Care Med 1995;23:646–651.
|
| 7 |
Thérond P, Bonnefont-Rousselot D, Davit-Spraul A, Conti M, Legrand A.
Biomarkers of oxidative stress: an analytical approach.
Curr Opin Clin Nutr Metab Care 2000;3:373–384.
|
| 8 |
Heyland DK, Dhaliwal R, Suchner U, Berger MM.
Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient.
Intensive Care Med 2005;31:327–337.
|
| 9 |
Crimi E, Sica V, Williams-Ignarro S, et al.
The role of oxidative stress in adult critical care.
Free Radic Biol Med 2006;40:398–406.
|
| 10 |
Duntas LH.
Selenium and inflammation: underlying anti-inflammatory mechanisms.
Horm Metab Res 2009;41:443–447.
|
| 11 |
McCowen KC, Malhotra A, Bistrian BR.
Stress-induced hyperglycemia.
Crit Care Clin 2001;17:107–124.
|
| 12 |
Zauner A, Nimmerrichter P, Anderwald C, et al.
Severity of insulin resistance in critically ill medical patients.
Metabolism 2007;56:1–5.
|
| 13 |
Van den Berghe G, Wilmer A, Milants I, et al.
Intensive insulin therapy in mixed medical/surgical intensive care units benefit versus harm.
Diabetes 2006;55:3151–3159.
|
| 14 |
Van den Berghe G, Wilmer A, Hermans G, et al.
Intensive insulin therapy in the medical ICU.
N Engl J Med 2006;354:449–461.
|
| 15 |
Van Den Berghe G, Wouters P, Weekers F, et al.
Intensive insulin therapy in critically ill patients.
N Engl J Med 2001;345:1359–1367.
|
| 16 |
Singh U, Jialal I.
Retracted: alpha-lipoic acid supplementation and diabetes.
Nutr Rev 2008;66:646–657.
|
| 17 |
Estrada DE, Ewart HS, Tsakiridis T, et al.
Stimulation of glucose uptake by the natural coenzyme α-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway.
Diabetes 1996;45:1798–1804.
|
| 18 |
Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A.
Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by α-lipoic acid in 3T3-L1 adipocytes.
Diabetologia 2000;43:294–303.
|
| 19 |
Henriksen EJ.
Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes.
Free Radic Biol Med 2006;40:3–12.
|
| 20 |
Saghaei M.
Random allocation software for parallel group randomized trials.
BMC Med Res Methodol 2004;4:26.
|
| 21 |
McClave SA, Martindale RG, Vanek VW, et al.
Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN).
JPEN J Parenter Enteral Nutr 2009;33:277–316.
|
| 22 |
Gleiter CH, Schug BS, Hermann R, Elze M, Blume HH, Gundert-Remy U.
Influence of food intake on the bio-availability of thioctic acid enantiomers.
Eur J clin Pharmacol 1996;50:513–514.
|
| 23 |
Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, Santaweesuk A.
Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alphalipoic acid: a randomized double-blinded placebo-controlled study.
Asia Pac J Clin Nutr 2012;21:12–21.
|
| 24 |
Galasko DR, Peskind E, Clark CM, et al.
Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures.
Arch Neurol 2012;69:836–841.
|
| 25 |
Preiser JC, Van Gossum A, Berré J, Vincent JL, Carpentier Y.
Enteral feeding with a solution enriched with antioxidant vitamins A, C, and E enhances the resistance to oxidative stress.
Crit Care Med 2000;28:3828–3832.
|
| 26 |
Crimi E, Liguori A, Condorelli M, et al.
The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial.
Anesth Analg 2004;99:857–863.
|
| 27 |
Namvaran F, Azarpira N, Rahimi-Moghaddam P, Dabbaghmanesh MH.
Polymorphism of peroxisome proliferator- activated receptor γ (PPARγ) Pro12Ala in the Iranian population: relation with insulin resistance and response to treatment with pioglitazone in type 2 diabetes.
Eur J Pharmacol 2011;671:1–6.
|
| 28 |
Zal F, Mostafavi‐Pour Z, Vessal M.
Comparison of the effects of vitamin E and/or quercetin in attenuating chronic cyclosporine A-induced nephrotoxicity in male rats.
Clin Exp Pharmacol Physiol 2007;34:720–724.
|
| 29 |
Hawker FH, Stewart PM, Snitch PJ.
Effects of acute illness on selenium homeostasis.
Crit Care Med 1990;18:442– 446.
|
| 30 |
Forceville X, Vitoux D, Gauzit R, Combes A, Lahilaire P, Chappuis P.
Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients.
Crit Care Med 1998;26:1536–1544.
|
| 31 |
Mishra V, Baines M, Perry SE, et al.
Effect of selenium supplementation on biochemical markers and outcome in critically ill patients.
Clin Nutr 2007;26:41–50.
|
| 32 |
Abilés J, de la Cruz AP, Castaño J, et al.
Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study.
Crit Care 2006;10:R146.
|
| 33 |
Cherubini A, Ruggiero C, Polidori MC, Mecocci P.
Potential markers of oxidative stress in stroke.
Free Radic Biol Med 2005;39:841–852.
|
| 34 |
Alexandrova ML, Bochev PG.
Oxidative stress during the chronic phase after stroke.
Free Radic Biol Med 2005;39:297–316.
|
| 35 |
Besler HT, Comoğlu S.
Lipoprotein oxidation, plasma total antioxidant capacity and homocysteine level in patients with multiple sclerosis.
Nutr Neurosci 2003;6:189– 196.
|
| 36 |
Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W.
Antioxidant status in patients with acute respiratory distress syndrome.
Intensive Care Med 1999;25:180–185.
|
| 37 |
Goraca A, Józefowicz-Okonkwo G.
Protective effects of early treatment with lipoic acid in LPS-induced lung injury in rats.
J Physiol Pharmacol 2007;58:541–549.
|
| 38 |
Cadirci E, Altunkaynak BZ, Halici Z, et al.
α-lipoic acid as a potential target for the treatment of lung injury caused by cecal ligation and puncture-induced sepsis model in rats.
Shock 2010;33:479–484.
|
| 39 |
Skibska B, Józefowicz-Okonkwo G, Goraca A.
Protective effects of early administration of alpha-lipoic acid against lipopolysaccharide-induced plasma lipid peroxidation.
Pharmacol Rep 2005;58:399–404.
|
| 40 |
Galley HF, Howdle PD, Walker BE, Webster NR.
The effects of intravenous antioxidants in patients with septic shock.
Free Radic Biol Med 1997;23:768–774.
|
| 41 |
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM.
alpha- lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential.
Biochim Biophys Acta 2009;1790:1149–1160.
|
| 42 |
Marangon K, Devaraj S, Tirosh O, Packer L, Jialal I.
Comparison of the effect of α-lipoic acid and α-tocopherol supplementation on measures of oxidative stress.
Free Radic Biol Med 1999;27:1114–1121.
|
| 43 |
Ansar H, Mazloom Z, Kazemi F, Hejazi N.
Effect of alpha- lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients.
Saudi Med J 2011;32:584–588.
|
| 44 |
Kamenova P.
Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid.
Hormones (Athens) 2006;5:251– 258.
|
| 45 |
Vidović B, Milovanović S, Dorđević B, et al.
Effect of alpha-lipoic acid supplementation on oxidative stress markers and antioxidative defense in patients with schizophrenia.
Psychiatr Danub 2014;26:205–213.
|
| 46 |
Evans JL, Goldfine ID, Maddux BA, Grodsky GM.
Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-Cell dysfunction?
Diabetes 2003;52:1–8.
|
| 47 |
Konrad D, Somwar R, Sweeney G, et al.
The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation.
Diabetes 2001;50:1464–1471.
|
| 48 |
Wiryana M.
The role of intensive insulin therapy in increasing superoxide dismutase (SOD) and normalizing hyperglycemia in critically ill patients.
Acta Med Indones 2009;41:59–65.
|
| 49 |
Pessin JE, Saltiel AR.
Signaling pathways in insulin action: molecular targets of insulin resistance.
J Clin Invest 2000;106:165–169.
|
| 50 |
Fink MP, Abraham E, Vincent JL, Kochanek P.
Textbook of Critical Care. 5th ed.
Philadelphia, PA: Elsevier Saunders; 2005.
|
| 51 |
Arabi YM, Tamim HM, Rishu AH.
Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality.
Crit Care Med 2009;37:2536–2544.
|
| 52 |
Griesdale DE, de Souza RJ, van Dam RM, et al.
Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data.
CMAJ 2009;180:821–827.
|
| 53 |
Heyland D, Muscedere J, Wischmeyer PE, et al.
A randomized trial of glutamine and antioxidants in critically ill patients.
N Engl J Med 2013;368:1489–1497.
|
| 54 |
Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK.
Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis.
Crit Care 2012;16:R66.
|