Abstract
Dexmedetomidine, an α-2 adrenergic receptor agonist, provides analgesia, sedation, anxiolysis, sympatholysis and anesthetic-sparing effect, without inducing signifi cant respiratory depression. Due to these properties, its clinical use is no longer limited to serving as a sedative agent in the intensive care unit. Proper airway management and the avoidance of cardiac and respiratory complications are common goals of everyday anesthesia practice. Ensuring airway safety is pivotal during the anesthesia stages of induction, maintenance and recovery. In this review, we focus on the advantages of dexmedetomidine in awake fi beroptic intubation (AFOI), diagnostic examinations and surgeries of patients with obstructed airways, and reducing emergence delirium effectively without causing further adverse events. With increasing implementation in different anesthetic scenarios, dexmedetomidine provides a favorable option to enhance patient safety and comfort.
Keywords
airway-related surgery; dexmedetomidine; DISE; intraoperative application; safety;
1.Introduction
Dexmedetomidine, a highly selective α-2 adrenoreceptor agonist, plays an increasingly important role in anesthetic practice of recent years. It exhibits dose-dependent sedation, anxiolysis, sympatholysis, anesthetic-sparing effects, and analgesia (acting on spinal and supraspinal sites) with minimal or no respiratory depression.1 Our aim is to review contemporary articles on the applications of dexmedetomidine in awake fi beroptic intubation (AFOI), reducing emergence delirium in airway-related surgeries, obese patients undergoing general anesthesia, and diagnostic procedure of the obstructed airway, along with our own clinical experiences.
2.Pharmacodynamics
Dexmedetomidine acts via binding to G-protein coupled α-2 adrenergic receptors, which are found in the central, peripheral, and autonomic nervous systems, as well as vital organs and blood vessels of the body. Three subtypes of α-2 adrenergic receptors (α- 2A, α-2B, α-2C) have been identifi ed, which exhibit different pharmacological functions and activities.2
The sedative effects are comparable to natural sleep in terms of respiratory pattern and electroencephalogram (EEG) changes. Dexmedetomidine is thought to activate an endogenous pathway which promotes non-rapid eye movement (NREM) sleep.3 Stimulation of α-2A receptor in the locus cerulerus inhibits noradrener- gic neurons, and disinhibits GABAnergic neurons in the ventrolateral preoptic neucleus (VLPO). The excepttion is the preservation of orexinergic neuron function in perifornical nucleus, which allows for arousal to occur.2,4 On the contrary, γ-aminobutyric acid (GABA)-related anesthetics (such as propofol and benzodiazepine) acts primary through enhancing the inhibitory effects of GABAnergic neurons in the VLPO, affecting normal sleep cycle, while norepinephrine release from the locus ceruleus remain unaffected.2 This is the major concern about GABA-related anesthetics on postoperative period, because the disruption of normal sleep pattern may then lead to cognitive dysfunction and postoperative delirium. Dexmedetomidine bypasses this GABAnergic pathway in the VLPO, thus able to provide anesthesia with lower incidences of emergence delirium and agitation, especially when volatile anesthetics are used.4,5
The analgesic effects of dexmedetomidine are mainly mediated by α-2A and α-2C receptors located in dorsal horn of the spinal cord. This results in reduced amplitude of nociception impulse transmission from peripheral to central brain, which reduces pain perception. For autonomic nerve system, activation of post-synaptic α-2 receptors in the sympathetic chain leads to sympatholysis, with accompanying hemodynamic presentation of hypotension and bradycardia.2
3.Pharmacokinetics
Dexmedetomidine has poor bioavailability due to its extensive first pass metabolism. As a result of unstable drug concentrations via oral intake, the intravenous route is preferred in perioperative and intensive care. When infused intravenously at a dose range of 0.2–0.7 mcg/kg/hr, it exhibits linear pharmacokinetics. It is rapidly distributed with an onset time of approximately 15 minutes and has an elimination half-life of 2 hours. The context sensitive half-time (t1/2CS) of dexmedetomidine during long-term infusion is increased with time (e.g. 10-minute infusion, t1/2CS 4 minutes; 8-hour infusion, t1/2CS 250 minutes.6 Dexmedetomidine is 94% protein-bound and does not displaces most of the protein-bound drugs commonly used in anesthesia. Hence, synergic use of dexmedetomidine with other anesthetic agents does not create competition to binding sites of plasma proteins. Concerning drug metabolism, dexmedetomidine is transformed to inactive metabolites through glucoronidation and cytochrome P450-mediated aliphatic hydroxylation. These inactive metabolites are then excreted from the body in urine (95%) and feces (4%).7 Dosage adjustment is required in patients with hepatic failure owning to lower metabolic rate.8 In addition, dexmedetomidine has higher inter-individual variability in clearance and volume of distribution. This variability is contributed by hypo-albuminemia, end-organ damage, changes in hemodynamics, and decreased cardiac output especially in the intensive care unit population.8
4.Anesthetic Sparing Effects
With increasing intraoperative use of dexmedetomidine, it has been found to reduce the dosage requirements of other anesthetic agents (both intravenous and inhaled agents)9,10 with an observed opioid sparing effect. Postoperative benefits have been reported with 25 to 54% reduction in the amount of “rescue” opioids used after surgery. Prolonged time for the first request of post-operative analgesics have also been seen when dexmedetomidine was used intraoperatively.11,12 Regardless of the methods of perioperative dexmedetomidine administration (i.e. as a bolus or continuous infusion), Bellon et al. found it still effectively reduced opioid consumption in the postoperative period.13 Sparing effects allows reduced dosage of anesthetic agents and opioids under the same therapeutic conditions, with the benefit of less dosage-related complications when dexmedetomidine is used in synergy with other anesthetics.
5.Adverse Effects
The most common adverse effects include hypotension (vasodilatation or diuresis from increased glomerular filtration), hypertension (transient), bradycardia, and complaints of dry mouth (decrease of salivation). Other miscellaneous effects such as fever, rigors, cyanosis, hyperglycemia, electrolyte imbalances, are extremely rare. Worth noting is that there have been few reports of dexmedetomidine causing arrhythmias,14,15 atrioventricular (AV) blocks,16 and even cardiac arrests.17 The mechanisms of the cardiac events are still now clear; there are few suggestions, for examples, dose-related intolerance,14 modulated vagal activity,15 depressive effect on sinoatrial and AV nodal function,16,18 inhibitory effect on cardiac pacing autoregulation16 and central sympathetic outflow (decreasing of norepinephrine release).17 Thus, patients with cardiac diseases or preexisting arrhythmias using dexmedetomidine must be use carefully monitored. In these cases, a dose reduction may be warranted.19
6.Intraoperative Applications
Facilitating AFOI
AFOI is widely accepted as the gold standard in management of known difficult airway, but it can be a terrible experience for the patient. Even with perfect airway nerve blockade, patients will go through horrific choking-like sensations and have violently uncontrolled reflex behaviors. These dangerous movements not only hurt themselves, but also disrupt proper airway management. A major challenge is how to provide adequate anxiolysis, whilst ensuring spontaneous ventilation. Conventional sedatives, like benzodiazepines, opioids, and propofol, may cause respiratory depression at higher doses or even lower doses due to inter-individual variation.
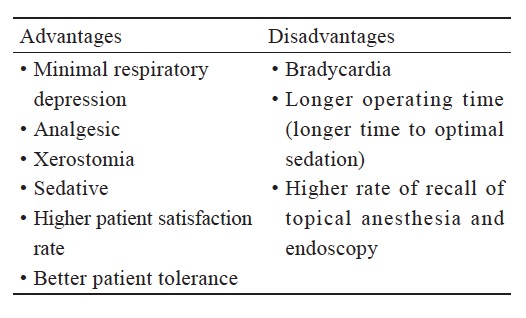
Dexmedetomidine’s properties make it an ideal solution to this problem, through providing a minimal respiratory inhibition and a relatively dry fibroscopic field for anesthesiologists due to its antisialogogue effect. Tsai et al. in 201020 compared dexmedetomidine (loading dose 1 mcg/kg over 10 min), with propofol (Ce 3 mcg/mL), and found that patients in the dexmedetomidine group showed better tolerance, lower incidences of hypoxia, and more cooperation. Their results revealed that a loading dose of dexmedetomidine (1 mcg/kg/hr) resulted in 50% of patients recalling the endoscopy insertion but only 5% recalling intubation. It showed higher levels of patient satisfaction than usual awake intubation without sedation. Zhou et al. in 201621 indicated that dexmedetomidine provides better sedation with similar success rates for intubation when compared to other conventional sedatives. Both studies stated that more local anesthetics must be applied in order to minimize cough reflex during fiberoptic intubation due to preservation of airway protection reflex. The higher risks of bradycardia and hypotension, can easily be managed with atropine and vasoactive agents. In a more recent study, it showed that a combination of dexmedetomidine with airway nerve blockade enhanced patient’s tolerance and comfort during awake intubation.22 In our own clinical practice, intravenous infusion of dexmedetomidine at a rate of 0.5–0.8 mcg/kg/hr for at least 20 minutes plus topical blocks can create optimal conditions for fiberoptic endotracheal intubation with less post-traumatic stress disorder (PTSD)-like memory recall.
However, dexmedetomidine did not provide better results when compared with remifentanil in several studies.23,24 For example, it takes longer time to adequate sedation level and decreased first success attempt rate.23 There was no significant difference in hemodynamic changes, adverse events, or patient’s tolerance. In a recent review, there is no strong evidence supporting that dexmedetomidine is the primary choice in AFOI, but is an additional option among other sedatives.25 The above descriptions are shown in the Table 1 .
Download full-size image
Reducing Incidences of Emergence Delirium in Airway-Related Surgeries
Generally, ear, nose, and throat (ENT) operations and other oral cavity surgeries under general anesthesia are associated with higher incidences of emergence agitation, which increases risks of self-extubation (causing hypoxia and aspiration pneumonia), negative pressure pulmonary edema, self-injury airway trauma, or injury of attending medical staffs.26 Other contributing risk factors are male gender, inhalational anesthesia, inadequate analgesia, and presence of urinary catheter or endotracheal tube. Meng et al. have demonstrated preventive effects of dexmedetomidine on emergence agitation and smooth extubation in the pediatric population undergoing tonsillectomy.27 In adults undergoing minor surgical procedure such as septoplasties, dexmedetomidine exhibits shorter time to extubation, and similar hemodynamic and recovery profile in comparison with remifentanil.28
However, a randomized control trial with patients undergoing orthognathic surgeries did not show a significant difference in emergence agitation compared with the control group;29 in this trial, dexmedetomidine was only given in a bolus dose (1 mcg/kg/hr) for 10 minutes at the end of surgery. The inadequate onset time was probably the reason for poor prophylactic result. Hence, several others have reported less emergence agitation with the use of dexmedetomidine (0.4 mcg/kg/hr) perioperatively in nasal surgery and in the elderly population receiving orthopedic surgery.28,30-32 Polat et al. compared remifentanil with dexmedetomidine and found significantly reduced incidences of emergence agitation in both groups (3.3 and 20%, respectively) compared with the placebo group (46.7%; p < 0.001).31 In order to reduce postoperative complications of emergence agitation, preventive use of dexmedetomidine has the potential to achieve the balance between providing sufficient sedation while preserving respiratory drive. In our experiences, continuous infusion of dexmedetomidine should be started immediately after anesthesia induction at a rate between 0.4–0.7 mcg/kg/hr. Due to less respiratory depression, dexmedetomidine can be continuously given until successful extubation. This facilitates a calm and comfortable experience for patients during transportation and in the post-anesthesia care unit. Physical restraint-related injuries can potentially be avoided.
One needs to keep in mind that post-surgical pain, endotracheal intubation, and inserted catheters also contribute to emergence agitation.26 Although dexmedetomidine exhibits opioid-sparing effect, some studies did not observe anti-delirium effects possibly due to the existence of other delirium-contributing factors.11 Thus, though dexmedetomidine can be used to reduce the risk of agitation, adequate pain control and early removal of foreign catheters are also important factors to ensure a comfortable and safe postoperative recovery in the post-anesthesia care unit.
Minimizing Risks of Adverse Respiratory Events in Obese Patients
When planning the anesthesia of obese patients, extra attention should be placed on obstructive sleep apnea (OSA), hypoventilation syndrome, and postoperative atelectasis. In the postoperative period following anesthesia, obese patients are prone to develop adverse respiratory events, referred to as critical respiratory events (CRE). Since dexmedetomidine experiences less respiratory depression, it can be utilized as a sedative agent in morbidly obese patient, as well as an anesthetic adjuvant during general anesthesia.33
In several studies, dexmedetomidine attenuates cardiovascular responses to noxious stimuli (such as intubation or surgical manipulations), along with a reduction of required dosage of opioid and volatile agents. Less opioid usage thereby lowers the incidence of postoperative nausea and vomiting (PONV).10,34-36 Abu-Halaweh et al. demonstrated that postoperative initiation of dexmedetomidine use (at a dosage of 0.3 mcg/kg/hr) reduced the mean total consumption of morphine following laparoscopic bariatric surgery.37 A meta-analysis by Singh et al.38 concluded that obese patients who receive perioperative dexmedetomidine had nearly one-third less opioids consumption in comparison with the controls and 25% reduction in the pain score. Most of the trials in this analysis used a dexmedetomidine infusion dose of 0.4 mcg/kg/min. The timing of the given dose on the opioid-sparing effect was higher when the infusions used in the postoperative phase in comparison with intraoperative phase alone. However, continuous infusion dexmedetomidine from intraoperative through postoperative phase was not compared by any presently studies. The incidence of PONV was reduced by 76% in comparison with controls, which is a clinical significant reduction without additional antiemetic drugs being used.38 This not only increased clinical safety but also likely to have better patient satisfaction. Although timing and dosing of dexmedetomidine is still controversial,38 in our clinical practice, intravenous infusion of dexmedetomidine (0.5 mcg/kg/hr) will be given 30 minutes before the end of surgery for obese patients with OSA who are at increased risks of compromised airway. Smooth extubation and maintenance of adequate respiratory drive are vital to ensure airway management safety in these high-risk patients. This result is comparable with a prospectively randomized-controlled trial by Tufanogullari et al. of recovery outcomes of bariatric surgeries. They found out that the time to tracheal extubation did not differ when comparing patients with and without dexmedetomidine infusion. In addition, the duration of post-anesthetic care unit (PACU) stay was significantly shorter in those with dexmedetomidine use intraoperatively.36
Enhancing Safety of Procedural Sedation of Drug-Induced Sleep Endoscopy (DISE)
In many recent reviews, dexmedetomidine has seen extensive use in non-intubated surgeries and other non-intubated procedures. It has been demonstrated able to be safely used in bronchoscopies, transesophageal echocardiographies, shockwave lithotripsies, vitreoretinal surgeries, tonsillectomies of pediatric patients, etc.9,39,40 We implement the routine use of dexmedetomidine to provide sedation for DISE, a dynamic airway evaluation exam for patients suffering from OSA.
The sedative agent used in DISE is to achieve rapid-eye movement stage of sleep, which is known to induce several levels of upper airway obstruction. Propofol and dexmedetomidine are the most used agents for this procedure.41 Generally, dexmedetomidine provides a better control of hemodynamic profile and bispectral index (BIS) value,42 has lesser degree of airway obstruction, pain level43 and desaturation. On the other hand, propofol has its own advantages, including it is more effective in rapid sedation,44 shorter half-life, and inducing greater degree of tongue obstruction;45 but has higher incidence of oxygen desaturation and inhibiting respiratory drive.44,46 Nevertheless, both dexmedetomidine and propofol could achieve similar patterns of airway obstruction and hemodynamic stability when the depth of sedation is controlled with BIS monitoring.47 In our clinical practice, a loading dose of 1 mcg/kg is given intravenously over 10 minutes to 15 minutes. The patients would fall asleep within a few minutes and the diagnostic endoscopic examination would begin. If the patient remains awake, an additional 5 minutes waiting time or extra rescue sedation, for example propofol 30–50 mg intravenously, will work to reach satisfactory anesthetic level for endoscopic examination. The most commonly observed adverse effects during DISE procedures are bradycardia and hypotension. In the absence of contraindications, atropine 0.1 mg/10 kg or glycopyrrolate 0.2 mg will be given intravenously. Even after the examination, profound bradycardia with hypotension can sometimes still occur in the post-anesthesia recovery room. Therefore, adequate hydration and increased staff alertness are necessary for perioperative care, with preparation of readily available inotropic agents.
7.Conclusion
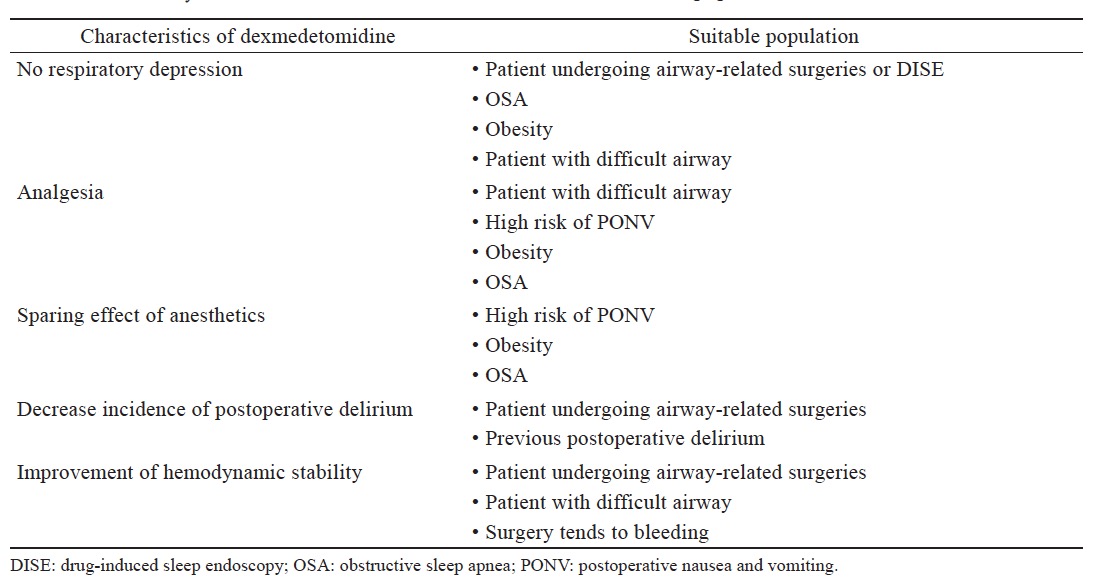
The new α-2 adrenergic receptor blocker dexmedetomidine is a promising drug with favorable anesthetic properties and potential for numerous clinical applications, beyond what we mentioned here (Table 2 ). Although there is a significant sympatholytic effect during dexmedetomidine infusions, hemodynamic changes can be safely managed and controlled with adequate fluid hydration and catecholamine use as indicated. With less respiratory depression, smoother emergence and generally more stable hemodynamic profile, dexmedetomidine not only provides a safer and more pleasant condition to perform procedures or surgeries involving difficult airway management, but also, improves overall quality of our perioperative anesthetic practice. Further novel uses of dexmedetomidine are constantly being developed, awaiting additional researches and randomized control trials to validate the results.
Download full-size image
References
| 1 |
Maze M, Scarfini C, Cavaliere F.
New agents for sedation in the intensive care unit.
Crit Care Clin 2001;17:881–898.
|
| 2 |
Panzer O, Moitra V, Sladen RN.
Pharmacology of sedative- analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral Mu antagonists.
Anesthesiol Clin 2011;29:587–605.
|
| 3 |
Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M.
Sleep quality of mechanically ventilated patients sedated with dexmedetomidine.
Intensive Care Med 2012;38:1982–1989.
|
| 4 |
Sanders RD, Maze M.
Contribution of sedative-hypnotic agents to delirium via modulation of the sleep pathway.
Can J Anaesth 2011;58:149–156.
|
| 5 |
Sanders RD, Maze M.
Noradrenergic trespass in anesthetic and sedative states.
Anesthesiology 2012;117:945–947.
|
| 6 |
Dyck JB, Maze M, Haack C, Azarnoff DL, Vuorilehto L, Shafer SL.
Computer-controlled infusion of intravenous dexmedetomidine hydrochloride in adult human volunteers.
Anesthesiology 1993;78:821–828.
|
| 7 |
Philipp M, Brede M, Hein L.
Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough.
Am J Physiol Regul Integr Comp Physiol 2002;283:R287–R295.
|
| 8 |
Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P.
Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine.
Clin Pharmacokinet 2017;56:893–913.
|
| 9 |
Naaz S, Ozair E.
Dexmedetomidine in current anaesthesia practice—a review.
J Clin Diagn Res 2014;8:GE01–GE04.
|
| 10 |
Bakhamees HS, El-Halafawy YM, El-kerdawy HM, Gouda NM, Altemyatt S.
Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass.
Middle East J Anaesthesiol 2007;19:537–551.
|
| 11 |
Jessen Lundorf L, Korvenius Nedergaard H, Møller AM.
Perioperative dexmedetomidine for acute pain after abdominal surgery in adults.
Cochrane Database Syst Rev 2016;2:CD010358.
|
| 12 |
Song J, Ji Q, Sun Q, Gao T, Liu K, Li L.
The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy.
J Neurosurg Anesthesiol 2016;28:14–20.
|
| 13 |
Bellon M, Le Bot A, Mchelet D, et al.
Efficacy of intraoperative dexmedetomidine compared with placebo for postoperative pain management: a meta-analysis of published studies.
Pain Ther 2016;5:63–80.
|
| 14 |
Bloor BC, Ward DS, Belleville JP, Maze M.
Effects of intravenous dexmedetomidine in humans. II.
Hemodynamic changes
|
| 15 |
Rao PB, Singh N, Ramalingam B.
Dexmedetomidine-induced atrial premature complex.
Ann Card Anaesth 2016;19:347–350.
|
| 16 |
Takata K, Adachi YU, Suzuki K, Obata Y, Sato S, Nishiwaki K.
Dexmedetomidine-induced atrioventricular block followed by cardiac arrest during atrial pacing: a case report and review of the literature.
J Anesth 2014;28:116– 120.
|
| 17 |
Bharati S, Pal A, Biswas C, Biswas R.
Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports.
Acta Anaesthesiol Taiwan 2011;49:165–167.
|
| 18 |
Sairaku A, Nakano Y, Suenari K, et al.
Dexmedetomidine depresses sinoatrial and atrioventricular nodal function without any change in atrial fibrillation inducibility.
J Cardiovasc Pharmacol 2016;68:473–478.
|
| 19 |
Chrysostomou C, Schmitt CG.
Dexmedetomidine: sedation, analgesia and beyond.
Expert Opin Drug Metab Toxicol 2008;4:619–627.
|
| 20 |
Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC.
A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation.
Anaesthesia 2010;65:254–259.
|
| 21 |
Zhou LJ, Fang XZ, Gao J, Zhangm Y, Tao LJ.
Safety and efficacy of dexmedetomidine as a sedative agent for performing awake intubation: a meta-analysis.
Am J Ther 2016;23:e1788–e1800.
|
| 22 |
Niyogi S, Basak S, Acharjee A, Chakraborty I.
Efficacy of intravenous dexmedetomidine on patient’s satisfaction, comfort and sedation during awake fibre-optic intubation in patients with cervical spondylotic myelopathy posted for elective cervical fixation.
Indian J Anaesth 2017;61:137–143.
|
| 23 |
Cattano D, Lam NC, Ferrario L, et al.
Dexmedetomidine versus remifentanil for sedation during awake fiberoptic intubation.
Anesthesiol Res Pract 2012;2012:753107.
|
| 24 |
Liu HH, Zhou T, Wei JQ, Ma WH.
Comparison between remifentanil and dexmedetomidine for sedation during modified awake fiberoptic intubation.
Exp Ther Med 2015;9:1259–1264.
|
| 25 |
He XY, Cao JP, He Q, Shi XY.
Dexmedetomidine for the management of awake fibreoptic intubation.
Cochrane Database Syst Rev 2014;(1):CD009798.
|
| 26 |
Yu D, Chai W, Sun X, Yao L.
Emergence agitation in adults: risk factors in 2,000 patients.
Can J Anaesth 2010;57:843–848.
|
| 27 |
Meng QT, Xia ZY, Luo T, et al.
Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study.
Int J Pediatr Otorhinolaryngol 2012;76:1036–1041.
|
| 28 |
Kim H, Ha SH, Kim CH, Lee SH, Choi SH.
Efficacy of intraoperative dexmedetomidine infusion on visualization of the surgical field in endoscopic sinus surgery.
Korean J Anesthesiol 2015;68:449–454.
|
| 29 |
Ham SY, Kim JE, Park C, Shin MJ, Shim YH.
Dexmedetomidine does not reduce emergence agitation in adults following orthognathic surgery.
Acta Anaesthesiol Scand 2014;58:955–960.
|
| 30 |
Kavalci G, Ethemoglu FB, Durukan P, Batuman A, Emre C.
Comparison of the effects of dexmedetomidine and remiphentanyl on emergence agitation after sevoflurane anesthesia in adults undergoing septoplasty operation: a randomized double-blind trial.
Eur Rev Med Pharmacol Sci 2013;17:3019–3023.
|
| 31 |
Polat R, Peker K, Baran I, Bumin Aydın G, Topçu Gülöksüz Ç, Dönmez A.
Comparison between dexmedetomidine and remifentanil infusion in emergence agitation during recovery after nasal surgery: a randomized double-blind trial.
Anaesthesist 2015;64:740–746.
|
| 32 |
Kim DJ, Kim SH, So KY, Jung KT.
Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery.
BMC Anesthesiol 2015;15:139.
|
| 33 |
Hofer RE, Sprung J, Sarr MG, Wedel DJ.
Anesthesia for a patient with morbid obesity using dexmedetomidine without narcotics.
Can J Anaesth 2005;52:176–180.
|
| 34 |
Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT.
Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis.
Br J Anaesth 2014;112:906– 911.
|
| 35 |
Gaszynski T, Czarnik K, Łaziński Ł, Gaszyński W.
Dexmedetomidine for attenuating haemodynamic response to intubation stimuli in morbidly obese patients anaesthetised using low-opioid technique: comparison with fentanyl- based general anaesthesia.
Anaesthesiol Intensive Ther 2016;48:275–279.
|
| 36 |
Tufanogullari B, White PF, Peixoto MP, et al.
Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables.
Anesth Analg 2008;106:1741–1748.
|
| 37 |
Abu-Halaweh S, Obeidat F, Absalom AR, et al.
Dexmedetomidine versus morphine infusion following laparoscopic bariatric surgery: effect on supplemental narcotic requirement during the first 24 h.
Surg Endosc 2016;30:3368–3374.
|
| 38 |
Singh PM, Panwar R, Borle A, Mulier JP, Sinha A, Goudra B.
Perioperative analgesic profile of dexmedetomidine infusions in morbidly obese undergoing bariatric surgery: a meta-analysis and trial sequential analysis.
Surg Obes Relat Dis 2017;13:1434–1446.
|
| 39 |
Afonso J, Reis F.
Dexmedetomidine: current role in anesthesia and intensive care.
Rev Bras Anestesiol 2012;62:118–133.
|
| 40 |
Mantz J, Josserand J, Hamada S.
Dexmedetomidine: new insights.
Eur J Anaesthesiol 2011;28:3–6.
|
| 41 |
Chang ET, Certal V, Song SA, et al.
Dexmedetomidine versus propofol during drug-induced sleep endoscopy and sedation: a systematic review.
Sleep Breath 2017;21:727–735.
|
| 42 |
Chattopadhyay U, Mallik S, Ghosh S, Bhattacharya S, Bisai S, Biswas H.
Comparison between propofol and dexmedetomidine on depth of anesthesia: a prospective randomized trial.
J Anaesthesiol Clin Pharmacol 2014;30:550–554.
|
| 43 |
Ma XX, Fang XM, Hou TN.
Comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during coblation- assisted upper airway procedure.
Chin Med J (Engl) 2012;125:869–873.
|
| 44 |
Kuyrukluyildiz U, Binici O, Onk D, et al.
Comparison of dexmedetomidine and propofol used for drug-induced sleep endoscopy in patients with obstructive sleep apnea syndrome.
Int J Clin Exp Med 2015;8:5691–5698.
|
| 45 |
Capasso R, Rosa T, Tsou DY, et al.
Variable findings for drug-induced sleep endoscopy in obstructive sleep apnea with propofol versus dexmedetomidine.
Otolaryngol Head Neck Surg 2016;154:765–770.
|
| 46 |
Cho JS, Soh S, Kim EJ, et al.
Comparison of three sedation regimens for drug-induced sleep endoscopy.
Sleep Breath 2015;19:711–717.
|
| 47 |
Yoon BW, Hong JM, Hong SL, Koo SK, Roh HJ, Cho KS.
A comparison of dexmedetomidine versus propofol during drug-induced sleep endoscopy in sleep apnea patients.
Laryngoscope 2016;126:763–767.
|