Abstract
Chronic post-surgical pain (CPSP) is a chronic pain condition, affecting many people who have undergone surgery. The development of CPSP is complex and the treatment options are limited; therefore, postsurgical pain management is crucial for satisfactory pain relief and prevention of CPSP development. In this review, we analyzed the existing treatment modalities for patients with CPSP. The treatments include opioids, acetaminophen, N-methyl-D-aspartate (NMDA) receptor antagonists, anticonvulsants, and local anesthesia. All treatments had signifi cant effect on CPSP and opioid-sparing effect; moreover, preventive analgesic treatment is important for improving quality of postoperative pain management. However, since most of the studies had limitations, further research on determining effective combinations and treatment modalities is required.
Keywords
chronic post-surgical pain (CPSP) ; enhanced recovery after surgery (ERAS) ; multimodal analgesia (MMA) ; preventive analgesia ; opioids ;
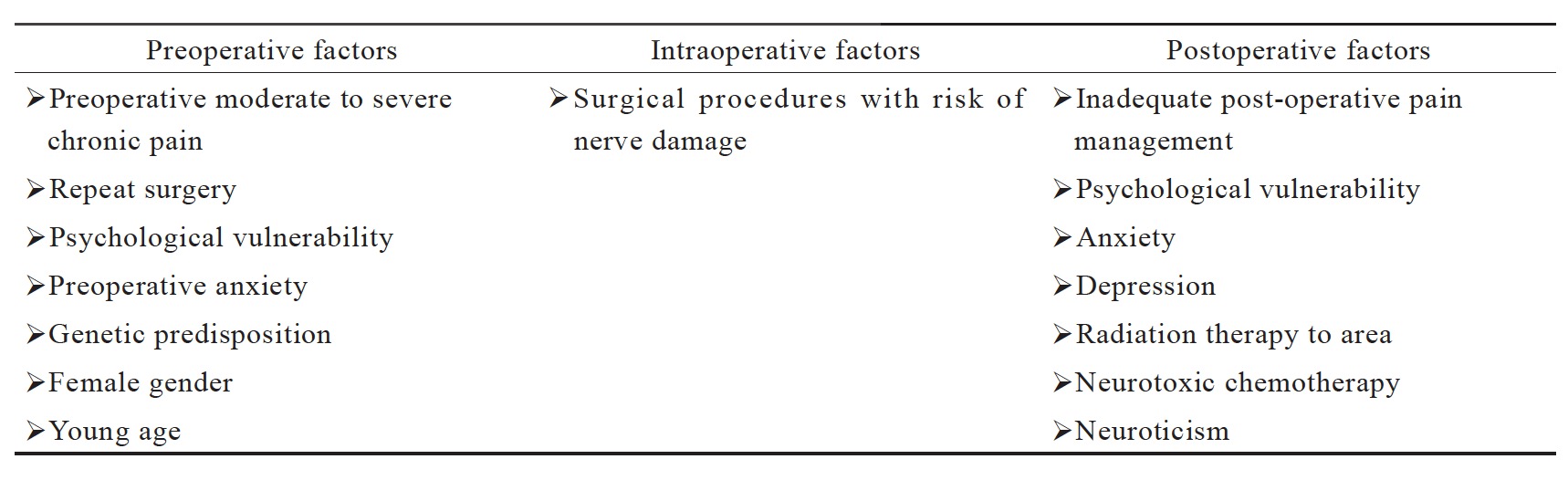
In Taiwan, total operations were approximately four million in 2015.2 Many procedures are likely to be associated with chronic post-surgical pain (CPSP), if the postoperative pain is not managed properly. CPSP is defined as persistent pain of longer than 2 months’ duration after the surgical procedure, excluding other causes of pain such as malignancy, infection, and pre-existing pain problem.2 The incidence rate of CPSP differs by surgery. In addition to the surgery type, numerous other risk factors may contribute to CPSP, including psychosocial factors, demographics, genetic predisposition, and pre-existing pain (Table 1).2
Download full-size image
Most studies have focused on amputation, breast surgery, thoracotomies, and inguinal hernia repair because these operations are considered to be associated with a combination of nociceptive and neuropathic symptoms and show high incidence of CPSP.3 The estimated incidence of CPSP following amputation is around 30%. Systematic reviews have summarized the frequency of CPSP after amputation; the latest published review that included data from five randomized controlled trials reported that moderate to severe chronic pain may occur in 80% of cases up to 20 years after amputation.4 Moreover, the incidence of persistent pain following mastectomy were reported to range from 13% to 69%.5 Post-mastectomy pain syndrome is attributable to surgical injury to the major peripheral nerves in the axilla during lymph node dissection.6,7 A recent prospective study found that breast implant and adjuvant radiotherapy were associated with increased CPSP.8 The incidence of chronic pain at 3- and 6-month post-thoracotomy was 57% and 47%, respectively.9 In a retrospective study, Pluijms and colleagues found an association between the severity of CPSP and extent of surgery.10 A recent trial showed that video-assisted thoracic surgery, with less nerve damage, had a lower incidence of CPSP and pain-related functional impairment.11 In a review by Aasvang, moderate-to-severe chronic groin pain and discomfort were reported in 10–12% of patients.12 Moreover, Bugada and colleagues found that in patients undergoing open inguinal hernia repair, CPSP was correlated with preoperative arterial hypertension.13
This article reviews the most recent literature on pathophysiology of CPSP and highlights the current understanding of etiologies and treatment options for perioperative management of postoperative pain.
Preemptive and Multimodal Analgesia (MMA)
Unrelieved CPSP is linked to adverse physiological changes that increase morbidity and mortality. To improve CPSP management, several concepts have been proposed, including preemptive analgesia and MMA.14 Central and peripheral sensitizations are the major causes of hypersensitivity to pain after injury.15 Wall proposed the concept of preemptive analgesia, whereby, preoperative local anesthesia and morphine block the induction of central sensitization, thus decreasing the incidence of hyperalgesia and postoperative pain intensity.16 However, in a review article, Møiniche et al. suggested that there was no superiority of preemptive analgesia administered before skin incision.17 Moreover, several randomized clinical trials comparing postoperative pain scores and total analgesic consumption, reported no significant effect in all outcome measures.18-21
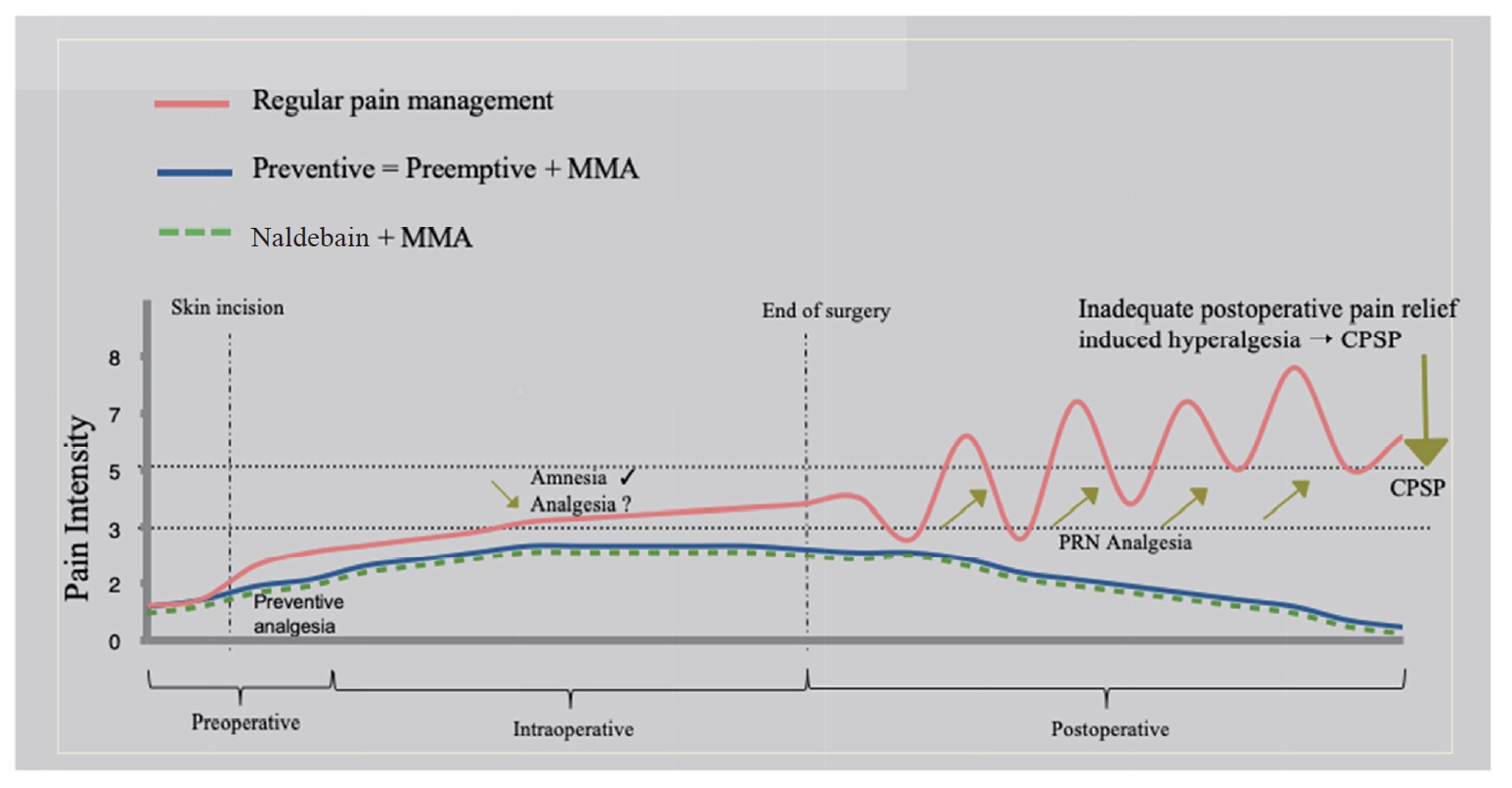
The aim of preventive analgesia is to decrease sensitization associated with noxious stimuli in the perioperative period;22 moreover, duration and efficacy of perioperative analgesic regimen are more important than preoperative timing of analgesic intervention (Fig. 1). Enhanced perioperative anesthesia protocols and use of peripheral nerve blocks were reported to improve patient satisfaction after hip arthroplasty and early postoperative rehabilitation.23,24 In a recent systematic review, preventive analgesia with intravenous and perineural dexamethasone was evaluated in 783 patients undergoing surgery; the result indicated that co-administration of dexamethasone with peripheral nerve block reduced postoperative pain and opioid requirement in the first 24 hours.25
Download full-size image
MMA is defined as administration of two or more analgesics or modalities through different mechanisms for postoperative analgesia.26 It improves postoperative pain management, has shorter duration hospital stay, and is an important management practice for enhanced recovery after surgery (ERAS).27 The American Society of Anesthesiologists and the American Pain Society recommend use of multimodal approach for postoperative pain control. The regimen may include a combination of opioids, acetaminophen, nonselective nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitor selective NSAIDs (COX-2 inhibitors), α-2 agonists (clonidine and dexmedetomidine), anticonvulsants (gabapentin and pregabalin), and local anesthetic techniques during the perioperative period via various routes.28 In this article, we review common analgesics and techniques for postoperative pain management, as well as prevention of CPSP.
Central neuraxial routes include epidural and spinal analgesia: co-administration of local anesthetic with opioid as single bolus dose or continuous administration via epidural catheter. Evidence supports epidural analgesia as standard procedure for major thoracic and abdominal procedures with satisfactory pain relief.29,30 The overall mortality was reduced by approximately one-third in patients who received neuraxial blockade for postoperative pain management with reduced cardiac and renal complications.31 Recently, a different mode for epidural analgesia was introduced. Capogna et al. reported that programmed intermittent epidural anesthetic bolus (PIEB) had less total local anesthetic consumption, fewer manual bolus doses for breakthrough pain, and greater patient satisfaction as compared to continuous epidural infusion (CEI).32 However, recent meta-analyses showed no reduction in patients’ mortality with perioperative epidural analgesia as compared with general anesthesia and systemic opioids for postoperative pain management.33 Moreover, contraindications and limitations were reported, such as spinal hematoma, epidural abscess, and technical complications, particularly in elderly patients with frequent antiplatelet treatment.34
Regional ultrasound-guided peripheral nerve blocks transversus abdominis plane (TAP), and paravertebral, brachial plexus or sciatic/femoral nerve blocks are increasingly incorporated into MMA regimens for post-surgical pain management. TAP block was reported to signifi cantly reduce morphine requirement and pain compared with placebo after abdominal surgery.35 Moreover, TAP block provides effective analgesia in patients in whom spinal anesthesia is contraindicated and reduces morphine consumption during the first 24 hours after surgery.36 A randomized triple-blind study reported that combined ilioinguinal-TAP block provided superior analgesia and reduced patient-controlled analgesia (PCA)-fentanyl dose and pain score.37
Surgical site infiltration with local anesthetics provides simple, effective, and inexpensive MMA. Systematic review of RCTs reported that continuous catheter-infusion of local anesthesia into the wound improved analgesia, reduced opioid consumption and related side effects, increased patient satisfaction, and reduced hospital stay; the action mechanism involved direct blocking of pain transmission in nociceptive afferents from the wound.38 Intra-articular analgesic infi ltration also reduced pain and opioid consumption after orthopedic procedures.39-41 In one study on patients with breast cancer, the wound infiltration group showed decreased CPSP and no indication for analgesia at 3- and 6-month follow-up.42 However, the risks associated with use of local anesthetics, including effects on the cardiac and central nervous system should be considered.
Pharmacology of Analgesics
Opioids are currently a major component in multimodal regimen. Intravenous or intramuscular administration of opioids has traditionally been used in management of moderate-to-severe pain. Intravenous PCA with opioid is widely used for postoperative analgesia. 28 A recent Cochrane review reported benefits of lower visual analog score (VAS) of pain symptom and increased patient satisfaction under PCA versus non-PCA.43 Studies showed that continuous basal infusion of opioid in naive patients may increase the likelihood of opioid-induced respiratory depression; however, it is unclear whether the continuous infusion is a significant risk factor for respiratory depression.44 The disadvantages of opioids include side effects such as confusion, sedation, hypotension, constipation, dizziness, pruritus, headache, nausea, vomiting, and respiratory depression. However, acute opioid tolerance and opioid-induced hyperalgesia (OIH) have been reported in the clinical use of opioids for anesthesia and postoperative pain management. Among possible action mechanisms, increased presynaptic glutamate release and postsynaptic N-methyl-D-aspartate (NMDA) receptor activation were suggested.45,46 A recent meta-analysis, including 1,494 patients, reported that high-dose intraoperative remifentanil was associated with development of OIH and high morphine requirement at > 1 day postoperatively.47 Therefore, selection of opioids should be based on pharmacological profile.
Nalbuphine is a semi-synthetic opioid used for moderate to severe pain relief, with agonist action on the κ-opioid receptor, as well as antagonist action on the μ-opioid receptor.48 The effect of low-dose nalbuphine may be similar to that of ultra-low-dose naloxone: inhibiting morphine-μ-opioid receptor interaction- induced side effects via Gi-protein signaling. Clinical-dose nalbuphine may inhibit morphine-induced side effects while maintaining analgesic effect through κ-opioid receptor agonism; moreover, at low dose, it may enhance the analgesic effect of morphine via Gs-protein, similar to ultra-low-dose naloxone.49,50 Nalbuphine showed same potency as that of morphine with few effects on cardiovascular hemodynamics in patients without cardiac disease;51 in addition, it provided safe and effective treatment for prehospital conditions.52 Yeh and colleagues reported that combined morphine and nalbuphine in PCA decreased the incidence of pruritus.53 Moreover, low-dose nalbuphine combined with NSAID had superior efficacy in elderly patients undergoing elective open gastrointestinal surgery in terms of postoperative analgesia and decreased severity of postoperative nausea and vomiting (PONV).54 In a current meta-analysis, the analgesic efficacy of nalbuphine provided a better safety profile than morphine in the aspect of certain side effects.55 Recently, naldebain, a slow-release nalbuphine prodrug with long duration of action, was newly introduced in Taiwan; it delivers and maintains an effective blood level for approximately 6 days. Naldebain intramuscularly at 24-hour preoperative is recommended for attaining peak plasma concentration and shows effective postoperative pain reduction.56 This regimen provides preemptive analgesia and continuous postoperative pain control for at least another 5 days. It shows potential as an ideal analgesic, providing preemptive analgesia, global perioperative pain processing coverage, and may be used in combination with either morphine or NSAID for management of minor-to-moderate postoperative pain and inhibition of CPSP development.
Acetaminophen is most widely used for postoperative pain with an opioid-sparing effect of approximately 20% for mild-to-moderate pain.57 Previous studies have demonstrated the effectiveness of intravenous acetaminophen in decreasing postoperative pain in patients undergoing various surgical procedures.58,59 A recent Cochrane review, including 75 studies, reported that intravenous acetaminophen offered at least 50% pain relief.60
Nonselective NSAID and selective COX-2 inhibitors decreased inflammation by diminishing cyclooxygenase activity and provided 30–50% opioid- sparing effect.57 Perioperative administration of ketorolac and COX-2 inhibitors significantly decreased the required dosage of intravenous opioid, shortened the gastrointestinal recovery, and reduced the patients’ hospital stay after gastric surgery.61,62
NMDA receptor antagonist ketamine and dextromethorphan, reduce neuroplasticity through inhibition of NMDA receptors. A systemic review reported sig- nificant reduction of CPSP at 3- and 6-months postoperatively, including thoracotomy, mastectomy, hysterectomy, and various orthopedic surgeries.63 However, Grady et al. reported that ketamine had no effect in improving postoperative analgesia or opioid-related side effects in patients who underwent abdominal hysterectomy.64
Gabapentinoids gabapentin and pregabalin, are anticonvulsants commonly used to treat neuropathic pain or fibromyalgia. The agents modulate central pain pathways through interactions with the α-2-delta subunit of voltage-dependent calcium channels. Evidence suggested that perioperative administration of gabapentin effectively reduced the incidence of CPSP.65 A meta-analysis confirmed that administration of pregabalin was associated with significant reduction of opioid consumption and neuropathic pain, but side effects of increased sedation, dizziness, and visual disturbance were reported.66
α-2 agonist dexmedetomidine, exerted antinociceptive effect through modulation of brain-derived neurotrophic factor (BDNF) located in the central nervous system and spinal cord.67 Systematic review and meta-analysis confirmed that intrathecal or epidural dexmedetomidine provided postoperative and neuropathic pain relief.68,69 A randomized controlled study indicated that addition of dexmedetomidine prolonged the action duration of interscalene brachial plexus block in arthroscopic shoulder surgery; however, it was associated with increased risk of hypotension and bradycardia.70 Dexmedetomidine is indicated for use in anesthesia induction and intraoperative infusion; however, it is not recommended in postoperative pain management regimen. In addition, in a recent study, intraoperative dexmedetomidine infusion effectively improved analgesia during and after elective laparoscopic cholecystectomy; it also significantly reduced postoperative morphine consumption and patient with severe postoperative pain and prolonged first rescue analgesic time.71
Melatonin-mediated analgesic effect possibly involves the β-endorphins, γ-aminobutyric acid (GABA) receptor, opioid receptors, and nitric oxide-arginine pathway.71 Early clinical trials suggested correlation between melatonin and chronic pain.72 Patients with chronic pain had significantly lowered melatonin levels in the blood and urine.73-75 Melatonin is also known to facilitate sleep. Particularly, pathological sleep disorder associated with sleep disturbance was reported in patients with chronic pain.76 Thus, melatonin has a potential role in postoperative pain management. A recent systematic meta-analysis suggested that melatonin could reduce oxidative stress and requirement of anesthetics.77 Likewise, our previous study showed that melatonin restored the antinociceptive effect of morphine through inhibition of spinal microglia activation in morphine-tolerant rats.78
Conclusions
Chronic pain after surgery is a common occurrence. Improved management of acute postoperative pain is important to prevent development of CPSP. Preemptive analgesia is reported to have significant analgesic benefit in animal models. Therefore, its timing of administration should be based upon pharmacokinetics and type of surgery. MMA and preemptive analgesia have advantages in terms of improving postoperative pain control, and minimizing opioid consumption. However, treatment of all patients with perioperative drugs under expectation of preventing conversion to chronic pain is impractical. Researchers are faced with the challenge to predict occurrence of CPSP and identify patients at risk for its development. Further research is needed to determine the modality, analgesic regimen, and timing and duration of perioperative pain management required for prevention of CPSP.
References
| 1 |
Yeh CC, Liao CC, Shih CC, Jeng LB, Chen TL.
Postoperative adverse outcomes among physicians receiving major surgeries: a nationwide retrospective cohort study.
Medicine (Baltimore) 2016;95:e4946.
|
| 2 | |
| 3 |
Humble SR, Dalton AJ, Li L.
A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy.
Eur J Pain 2015;19:451–465.
|
| 4 | |
| 5 |
Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH.
Neuropathic pain following breast cancer surgery: proposed classification and research update.
Pain 2003;104:1–13
|
| 6 |
Kudel I, Edwards RR, Kozachik S, et al.
Predictors and consequences of multiple persistent postmastecto-my pains.
J Pain Symptom Manage 2007;34:619–627.
|
| 7 |
Kehlet H, Jensen TS, Woolf CJ.
Persistent postsurgical pain: risk factors and prevention.
Lancet 2006;367:1618–1625.
|
| 8 |
Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M.
Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: a double-blind, placebo-controlled randomized trial.
Pain Physician 2015;18:E139–E146.
|
| 9 |
Bayman EO, Brennan TJ.
Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis.
J Pain 2014;15:887–897.
|
| 10 |
Pluijms WA, Steegers MA, Verhagen AF, Scheffer GJ, Wilder-Smith OH.
Chronic post-thoracotomy pain: a retrospective study.
Acta Anaesthesiol Scand 2006;50:804–808.
|
| 11 |
Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Kehlet H.
Persistent postsurgical pain after video-assisted thoracic surgery—an observational study.
Acta Anaesthesiol Scand 2016;60:650–658.
|
| 12 |
Aasvang E, Kehlet H.
Chronic postoperative pain: the case of inguinal herniorrhaphy.
Br J Anaesth 2005;95:69–76.
|
| 13 |
Bugada D, Lavand’homme P, Ambrosoli AL, et al.
Effect of preoperative inflammatory status and comorbidities on pain resolution and persistent postsurgical pain after inguinal hernia repair.
Mediators Inflamm 2016;2016:5830347.
|
| 14 |
Katz J, Clarke H, Seltzer Z.
Review article: preventive analgesia: quo vadimus?
Anesth Analg 2011;113:1242–1253.
|
| 15 |
Latremoliere A, Woolf CJ.
Central sensitization: a generator of pain hypersensitivity by central neural plasticity.
J Pain 2009;10:895–926.
|
| 16 | |
| 17 |
Møiniche S, Kehlet H, Dahl JB.
A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia.
Anesthesiology 2002;96:725–741.
|
| 18 |
Gordon SM, Brahim JS, Dubner R, McCullagh LM, Sang C, Dionne RA.
Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period.
Anesth Analg 2002;95:1351–1357, table of contents.
|
| 19 |
Katz J, Cohen L.
Preventive analgesia is associated with reduced pain disability 3 weeks but not 6 months after major gynecologic surgery by laparotomy.
Anesthesiology 2004;101:169–174.
|
| 20 |
Ong CK, Lirk P, Seymour RA, Jenkins BJ.
The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis.
Anesth Analg 2005;100:757–773, table of contents.
|
| 21 |
Klasen J, Haas M, Graf S, et al.
Impact on postoperative pain of long-lasting pre-emptive epidural analgesia before total hip replacement: a prospective, randomised, double-blind study.
Anaesthesia 2005;60:118–123.
|
| 22 |
Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE.
Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty.
A randomized controlled trial. J Bone Joint Surg Am 2009;91:29–37.
|
| 23 |
Green C, Byrne AM, O’Loughlin P, Molony D, Harmon D, Masterson E.
Surgeon delivered psoas compartment block in total hip arthroplasty.
J Arthroplasty 2014;29:393–396.
|
| 24 |
Heesen M, Klimek M, Imberger G, Hoeks SE, Rossaint R, Straube S.
Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis.
Br J Anaesth 2018;120:212–227.
|
| 25 | |
| 26 |
Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management.
Anesthesiology 2012;116:248–273.
|
| 27 |
Radresa O, Chauny JM, Lavigne G, Piette E, Paquet J, Daoust R.
Current views on acute to chronic pain transition in post-traumatic patients: risk factors and potential for pre-emptive treatments.
J Trauma Acute Care Surg 2014;76:1142–1150.
|
| 28 |
Chou R, Gordon DB, de Leon-Casasola OA, et al.
Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council.
J Pain 2016;17:131–157.
|
| 29 |
Rawal N.
Current issues in postoperative pain management.
Eur J Anaesthesiol 2016;33:160–171.
|
| 30 |
Svircevic V, van Dijk D, Nierich AP, et al.
Meta-analysis of thoracic epidural anesthesia versus general anesthesia for cardiac surgery.
Anesthesiology 2011;114:271–282.
|
| 31 |
Rodgers A, Walker N, Schug S, et al.
Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials.
BMJ 2000;321:1493.
|
| 32 |
Capogna G, Camorcia M, Stirparo S, Farcomeni A.
Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome.
A randomized double-blind study in nulliparous women. Anesth Analg 2011;113:826–831.
|
| 33 |
Rawal N.
Epidural technique for postoperative pain: gold standard no more?
Reg Anesth Pain Med 2012;37:310–317.
|
| 34 |
Ding X, Jin S, Niu X, Ren H, Fu S, Li Q.
A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis.
PLoS One 2014;9:e96233.
|
| 35 |
Johns N, O’Neill S, Ventham NT, Barron F, Brady RR, Daniel T.
Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis.
Colorectal Dis 2012;14:e635–e642.
|
| 36 |
Abdallah FW, Halpern SH, Margarido CB.
Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anaesthesia? A systematic review and meta-analysis.
Br J Anaesth 2012;109:679–687.
|
| 37 |
Staker JJ, Liu D, Church R, et al.
A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section.
Anaesthesia 2018;73:594–602.
|
| 38 |
Liu SS, Richman JM, Thirlby RC, Wu CL.
Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials.
J Am Coll Surg 2006;203:914–932.
|
| 39 |
Wang X, Xiao L, Wang Z, Zhao G, Ma J.
Comparison of peri-articular liposomal bupivacaine and standard bupivacaine for postsurgical analgesia in total knee arthroplasty: a systematic review and meta-analysis.
Int J Surg 2017;39:238–248.
|
| 40 |
Acosta-Olivo C, Tamez-Mata Y, Murillo-Rodriguez J, Peña-Martínez V, Villa-Chavarría J.
Intrarticular infiltration of bupivacaine and magnesium sulfate in distal radius fractures.
A pilot study. Acta Ortop Mex 2017;31:217–221. [In Spanish, English/Spanish abstract]
|
| 41 |
Shin JJ, McCrum CL, Mauro CS, Vyas D.
Pain management after hip arthroscopy: systematic review of randomized controlled trials and cohort studies.
Am J Sports Med 2018;46:3288–3298.
|
| 42 |
Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C.
Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer.
Anesth Analg 2005;101:1427–1432.
|
| 43 |
McNicol ED, Ferguson MC, Hudcova J.
Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain.
Cochrane Database Syst Rev 2015;(6):CD003348.
|
| 44 |
George JA, Lin EE, Hanna MN, et al.
The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis.
J Opioid Manag 2010;6:47–54.
|
| 45 |
Chu LF, Angst MS, Clark D.
Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations.
Clin J Pain 2008;24:479–496.
|
| 46 |
Gu X, Wu X, Liu Y, Cui S, Ma Z.
Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine.
Mol Pain 2009;5:76.
|
| 47 |
Fletcher D, Martinez V.
Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis.
Br J Anaesth 2014;112:991–1004.
|
| 48 |
Zeng Z, Lu J, Shu C, et al.
A comparison of nalbuphine with morphine for analgesic effects and safety: meta-analysis of randomized controlled trials.
Sci Rep 2015;5:10927.
|
| 49 |
Wong CS.
The mechanisms of ultra-low dose opioid agonist-antagonist.
J Formos Med Assoc 2011;110:666; author reply 667.
|
| 50 |
Chou KY, Tsai RY, Tsai WY, et al.
Ultra-low dose (+)-naloxone restores the thermal threshold of morphine tolerant rats.
J Formos Med Assoc 2013;112:795–800.
|
| 51 |
Miller RR.
Evaluation of nalbuphine hydrochloride.
Am J Hosp Pharm 1980;37:942–949.
|
| 52 |
Stene JK, Stofberg L, MacDonald G, Myers RA, Ramzy A, Burns B.
Nalbuphine analgesia in the prehospital setting.
Am J Emerg Med 1988;6:634–639.
|
| 53 |
Yeh YC, Lin TF, Lin FS, Wang YP, Lin CJ, Sun WZ.
Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain.
Br J Anaesth 2008;101:542–548.
|
| 54 |
Mao Y, Cao Y, Mei B, et al.
Efficacy of nalbuphine with flurbiprofen on multimodal analgesia with transverse abdominis plane block in elderly patients undergoing open gastrointestinal surgery: a randomized, controlled, double-blinded trial.
Pain Res Manag 2018;2018:3637013.
|
| 55 |
Sadafule NN, Karhade SS.
Comparative study of efficacy of preoperative nalbuphine hydrochloride and pentazocine lactate on hemodynamic response to tracheal intubation and postoperative analgesia.
Anesth Essays Res 2018;12:218–222.
|
| 56 |
Tien YE, Huang WC, Kuo HY, et al.
Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation.
Biopharm Drug Dispos 2017;38:494–497.
|
| 57 |
Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N.
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review.
Br J Anaesth 2011;106:292–297.
|
| 58 |
Salihoglu Z, Yildirim M, Demiroluk S, et al.
Evaluation of intravenous paracetamol administration on postoperative pain and recovery characteristics in patients undergoing laparoscopic cholecystectomy.
Surg Laparosc Endosc Percutan Tech 2009;19:321–323.
|
| 59 |
Wininger SJ, Miller H, Minkowitz HS, et al.
A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery.
Clin Ther 2010;32:2348–2369.
|
| 60 |
Stott A.
Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain.
Nurs Stand 2017;31:42–43.
|
| 61 |
Lohsiriwat V.
Opioid-sparing effect of selective cyclooxygenase- 2 inhibitors on surgical outcomes after open colorectal surgery within an enhanced recovery after surgery protocol.
World J Gastrointest Oncol 2016;8:543–549.
|
| 62 |
Lu CH, Liu JY, Lee MS, et al.
Preoperative cotreatment with dextromethorphan and ketorolac provides an enhancement of pain relief after laparoscopic-assisted vaginal hysterectomy.
Clin J Pain 2006;22:799–804.
|
| 63 |
McNicol ED, Schumann R, Haroutounian S.
A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain.
Acta Anaesthesiol Scand 2014;58:1199–1213.
|
| 64 |
Grady MV, Mascha E, Sessler DI, Kurz A.
The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy.
Anesth Analg 2012;115:1078–1084.
|
| 65 |
Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J.
The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis.
Anesth Analg 2012;115:428–442.
|
| 66 |
Mishriky BM, Waldron NH, Habib AS.
Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis.
Br J Anaesth 2015;114:10–31.
|
| 67 |
Dai S, Qi Y, Fu J, et al.
Dexmedetomidine attenuates persistent postsurgical pain by upregulating K+-Cl− cotransporter-2 in the spinal dorsal horn in rats.
J Pain Res 2018;11:993–1004.
|
| 68 |
Chan AK, Cheung CW, Chong YK.
Alpha-2 agonists in acute pain management.
Expert Opin Pharmaco 2010;11:2849–2868.
|
| 69 |
Blaudszun G, Lysakowski C, Elia N, Tramèr MR.
Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials.
Anesthesiology 2012;116:1312–1322.
|
| 70 |
Jung HS, Seo KH, Kang JH, Jeong JY, Kim YS, Han NR.
Optimal dose of perineural dexmedetomidine for interscalene brachial plexus block to control postoperative pain in patients undergoing arthroscopic shoulder surgery: a prospective, double-blind, randomized controlled study.
Medicine (Baltimore) 2018;97:e0440.
|
| 71 |
Marseglia L, D’Angelo G, Manti S, et al.
Analgesic, anxiolytic and anaesthetic effects of melatonin: new potential uses in pediatrics.
Int J Mol Sci 2015;16:1209–1220.
|
| 72 |
Srinivasan V, Pandi-Perumal SR, Spence DW, et al.
Potential use of melatonergic drugs in analgesia: mechanisms of action.
Brain Res Bull 2010;81:362–371.
|
| 73 |
Wikner J, Hirsch U, Wetterberg L, Röjdmark S.
Fibromyalgia—a syndrome associated with decreased nocturnal melatonin secretion.
Clin Endocrinol (Oxf) 1998;49:179–183.
|
| 74 |
Masruha MR, de Souza Vieira DS, Minett TS, et al.
Low urinary 6-sulphatoxymelatonin concentrations in acute migraine.
J Headache Pain 2008;9:221–224.
|
| 75 |
Cutolo M, Maestroni GJ.
The melatonin-cytokine connection in rheumatoid arthritis.
Ann Rheum Dis 2005;64:1109–1111.
|
| 76 |
Srinivasan V, Cardinali DP, Srinivasan US, et al.
Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection.
Ther Adv Neurol Disord 2011;4:297–317.
|
| 77 |
Andersen LP, Werner MU, Rosenberg J, Gögenur I.
A systematic review of peri-operative melatonin.
Anaesthesia 2014;69:1163–1171.
|
| 78 |
Lin SH, Huang YN, Kao JH, Tien LT, Tsai RY, Wong CS.
Melatonin reverses morphine tolerance by inhibiting microglia activation and HSP27 expression.
Life Sci 2016;152:38–43.
|