Abstract
Objective
The aim of this meta-analysis is to verify the effectiveness of postoperative pain relief by perioperative parecoxib, a selective intravenous cyclooxygenase-2 (COX-2) inhibitor, for patients receiving laparoscopic cholecystectomy (LC) surgery.
Methods
Electronic databases were searched for the randomized controlled trials (RCTs) to evaluate the effectiveness of pain relief by parecoxib or placebo for patients receiving LC surgery. The primary outcomes was the pain score evaluation using visual analogue scale (VAS). The secondary outcomes were the opioids consumption or analgesic supplement requirement and incidence of any adverse events such as dizziness, nausea and vomiting.
Results
Ten trials with 916 patients were included. Perioperative parecoxib significantly reduced postoperative pain score with rest at post-anaesthesia care unit (PACU; mean difference [MD] = -0.58, 95% confi dence interval [CI] = -1.04 to -0.12, p = 0.01) after LC. Postoperative opioid requirement or analgesic supplement for rescue of pain was also effectively reduced (relative risk [RR] = 0.47, 95% CI = 0.33 to 0.66, p < 0.0001). The incidence of side effects such as postoperative nausea and vomiting (PONV) was unaffected (RR=0.83, 95% CI=0.63 to 1.10, p=0.20).
Conclusion
Perioperative intravenous parecoxib could effectively provide pain relief and reduced postoperative morphine consumption or rescue analgesics for LC surgery without causing additional adverse concerns.
Keywords
COX-2 inhibitor ; parecoxib ; laparoscopic cholecystectomy ; pain relief ; meta-analysis ;
Introduction
Laparoscopic cholecystectomy (LC) has become the world trend to be the surgical solution for various pathologies of gall bladder diseases due to relatively less invasive, less postoperative complications with prompt recovery after surgery.1,2 However, discomfort and wound pain after surgery are still annoying and making the day-surgery unacceptable for most patients.3
In order to solve the issue of pain relief after surgery, many pain relief modalities have been suggested such as opioids, non-opioids analgesics, non-steroid anti-inflammatory drugs (NSAIDs), or cyclooxygenase-2 (COX-2) inhibitors.4-6 Morphine-like analgesics provide effective analgesia and being easy to apply through different routes such as intravenous, intramuscular or oral tablets for the patients. However, various extent of conscious disturbance, nausea, vomiting, pruritus and urination difficulty due to opioids still occur in high incidence and hinder their application in most cases.7 Non-opiods analgesics, such as acetaminophen, provide both anti-inflammatory and analgesic effects. While, their liver and renal toxicity cause major concerns for their clinical applications, esp. in cases receiving LC with abnormal liver function.8
NSAID, such as selective intravenous COX-2 inhibitor, parecoxib, has been suggested to be an effective alternative providing both pain-relief and anti- inflammation in various surgeries.9-11 Although they have been linked to increased cardiovascular events such as heart attacks, broad spectrum of its clinical application still been suggested.12,13 Parecoxib, oral following intravenous form, has been proposed for pain relief for the LC.14 However, its effectiveness or side effects when applying for postoperative pain relief for the patients receiving LC was still controversial. 15-17
The aim of this study is to verify the effectiveness as well as the adverse events of intravenous selective COX-2 inhibitor, parecoxib, for LC through the meta- analysis of search of various database. Therefore, this systematic review and meta-analysis focuses on whether perioperative intravenous parecoxib could provide effective postoperative pain-relief without significant side effects for patients receiving LC.
Methods
Sources
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 Review protocol has been registered in International Prospective Register of Systematic Reviews (PROSPERO; No. CRD42018102905) before the study.
Inclusion and Exclusion Criteria
Two independent reviewers (Yu-Cih Lin and Chien-Yu Chen) screened duplicatedly for all articles of randomized controlled trials (RCTs) evaluating the effect of postoperative pain-relief as well as outcomes of interest (incidence of drug-related adverse effects) when using perioperative intravenous parecoxib for patients receiving LC. We excluded: (1) interventions delivered through oral, intramuscular or neuroaxial routes; (2) different regimens or doses of parecoxib given between the experiment and the control; (3) parecoxib given together with other analgesics; (4) non-elective surgery; (5) duplicate reporting of patient cohorts.
Search Strategy and Study Selection
Comprehensive literature search in databases including PubMed, EMBASE, the Cochrane Library databases, Google Scholar and the ClinicalTrials.gov registry (http://clinicaltrials.gov) was performed independently by Yu-Cih Lin and Chien-Yu Chen. Relevant keywords applied for medical subject headings and free text searches were selective COX-2 inhibitor, parecoxib, LC, postoperative pain relief. There was no language restriction in the PubMed searching through June 2018 for the related citations.
Data Extraction
Two reviewers (Yu-Cih Lin and Chien-Yu Chen) extracted the baseline as well as all outcome data, including study design, participant data, inclusion and exclusion criteria, evaluation of pain-relief by pain scale, dosage of adopted drug, timing of prescription, drugs and dosage for analgesic supplement, and any resulting complication. If an agreement could not be reached, the dispute was resolved with the help of a third reviewer (Chuen-Chau Chang).
Methodological Quality Appraisal
The quality of each study was assessed based on the adequacy of randomization, the allocation concealment, the blinding of the patients and the outcome assessors, the length of the follow-up period, the reporting of study withdrawals, the performance of an intention-to-treat analysis, and other potential bias assessed using Cochrane Collaboration’s tool.19 Disagreements existed among subtracted data were adjudicated by a third reviewer (Chuen-Chau Chang).
Outcomes and Statistical Analysis
The primary outcome was the effectiveness of postoperative pain relief defined by the scoring of visual analogue scale (VAS). The secondary outcomes included the postoperative need for supplementary analgesics that about postoperative cumulative morphine equivalent dose or additional analgesics needed, and related complications such as nausea, vomiting, and dizziness. We conducted meta-analysis with the Review Manager, version 5.3 (Cochrane Collaboration, Oxford, England), and Comprehensive Meta Analysis Version 2 (Biostat, Englewood, NJ, USA) for publication bias. A random-effects model was used to calculate the pooled estimates of adjusted mean differeence (MD) or relative risk (RR). Standard deviations were estimated from the confidence interval (CI) limit, and the standard error or range values were provided from studies cited. The effect sizes of continuous outcomes were reported as MD or dichotomous outcomes were reported as RR and the precision value based on a 95% CI. A pooled estimate of value was calculated using the DerSimonian and Laird random-effects model.20 The statistical heterogeneity and the inconsistency of treatment effects across the studies was evaluated by applying the Cochrane Q test and I2 statistics, respectively. Statistical significance was set at 0.10 for the Cochrane Q test. The Egger test was used to assess the funnel plot for significant asymmetry, indicating possible publication or other bias.21 The “trim and fill” method was used to test and adjust publication bias.22,23
Sensitivity Analyses
We conducted sensitivity analyses to evaluate methodological quality, postoperative patient-controlled analgesia (PCA) use or not and surgical techniques to test the stability of the integration effect and to assess statistical heterogeneity. Regarding the quality assessment, bias of selection, performance, detection and attrition were excluded. If random sequence generation and allocation concealment were both unclearly reported or any were categorized as high-risk, we counted this as selection bias.
Results
Search Results and Study Description
Among 176 initially evaluated abstracts, 111 studies met the initial inclusion criteria (Fig. 1), 65 trials were subsequently excluded due to duplicate citations and 77 studies further being excluded after reading titles and abstracts. There are 34 articles were retrieved for further evaluation. Among them, two trials were mixed with other surgeries, five trials were combined with valdecoxib, three trials were using different COX-2 inhibitor, etoricoxib, one trial with rofecoxib, one trail with valdecoxib, three trials with celecoxib, and four trials with rofecoxib, two trial had no placebo or control group, three trials did not have raw data. Overall, 10 studies were included in the qualitative synthesis.
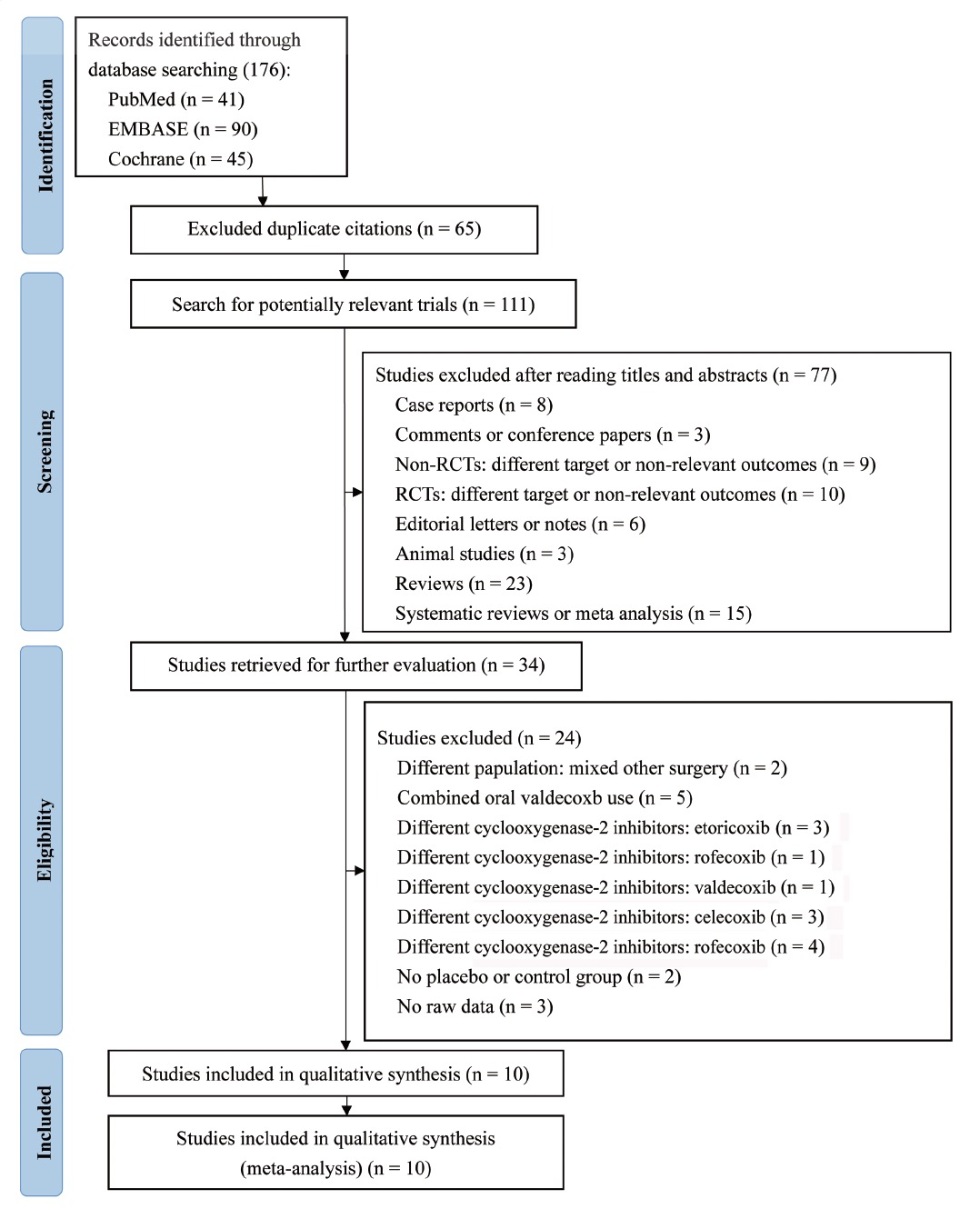
Download full-size image
Published from 2006 to 2014, the characteristics of the 10 RCTs with 916 participants were shown in Table 1.15-17, 24-30 One study was published in simplified Chinese,26 and the remaining nine were published in English. The sample sizes ranged from 45 to 180 patients. There are five RCTs of patients receiving three or four port LC,15,24,26,27,29 other five stydies were unclear-port LC,16,17,25,28,30 two trials were used PCA analgesia machine for postoperative pain control,17,24 and the other seven trials were used bolus opioids as the rescue drug,15,16,25-29 only one trial was not used any drug to control postoperative pain.30 The average age of patients ranged 38.0–64.1 years. The American Society of Anesthesiologists (ASA) Physical Status of all participants is I–II or I–III.
Most studies have a dose of 40 mg parecoxib per injection, and only two studies have different doses each time, one for each dose of 20 mg25 and the other for 0.8 mg per kg.16 Two studies injected the parecoxib 30 minutes before surgery,26,27 four trials were given the parecoxib before induction anesthesia,15,25,28,30 and other four studies were given the parecoxib at postoperative 0 hour.16,17,24,29 Three trials, parecoxib was given again 12 hours after surgery,17,26,27 in one of the trials, parecoxib was given again at 36 hours and 48 hours after surgery.26 One study was injected the parecoxib again at 12 hours after the first dose,25 another trial, parecoxib was given again when gallbladder was removed.28 One study investigated various doses of percoxib,24 and other trial investigated different times of injected parecoxib.28 Patient characteristics, postoperative pain control techniques and surgical procedures are summarized in Table 1.
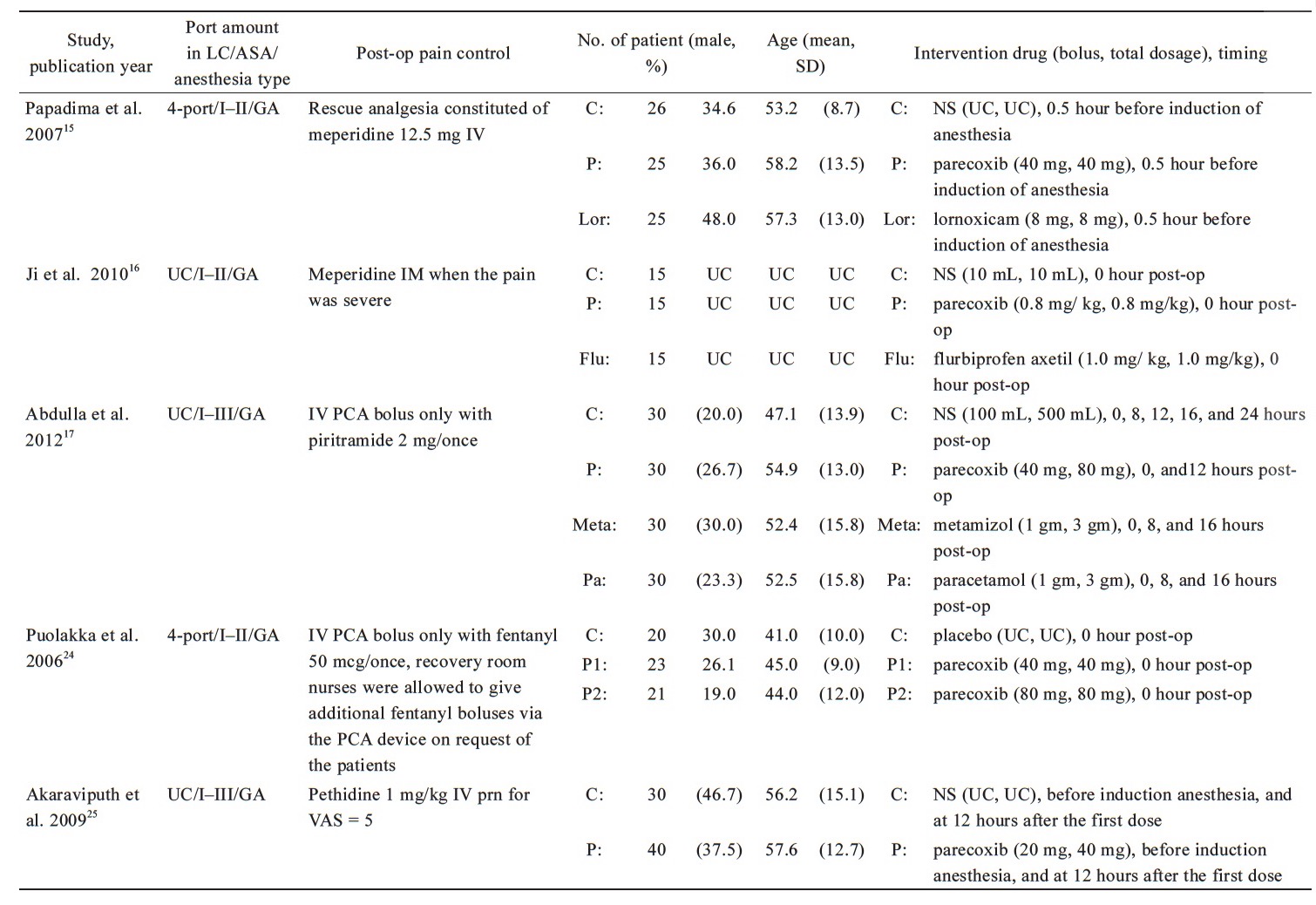
Download full-size image
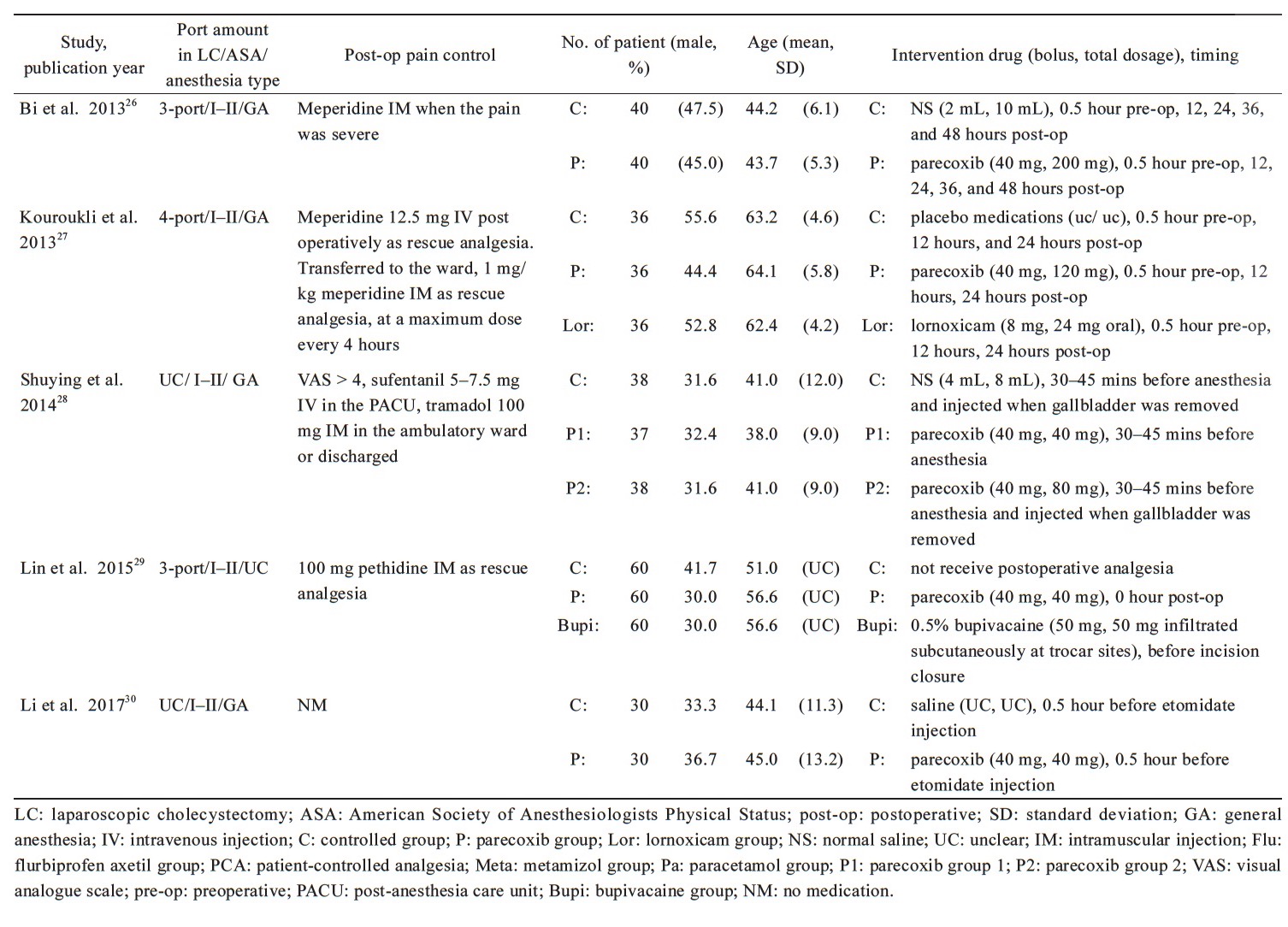
Download full-size image
Quality Assessment of Methodology
The quality assessment of RCT methodology is shown in Table 2. Acceptable sequence generation was noted in six studies,17,24,27-30 while four trials were unclear.15,16,25,26 How allocation concealment was carried out was clearly described in three RCTs,24,25,30 and the rest were unclear. Performance bias was unclear in three studies,16,25,26 while detection bias was identified unclear in five trials.15,16,24,26,29 One study was found attrition bias unclear.25 Two studies performed a per-protocol analysis, and 16 patients were withdrawn in total during the follow-up periods,24,28 another one study has identified have reporting bias and other biases included differences in dose per injection.25
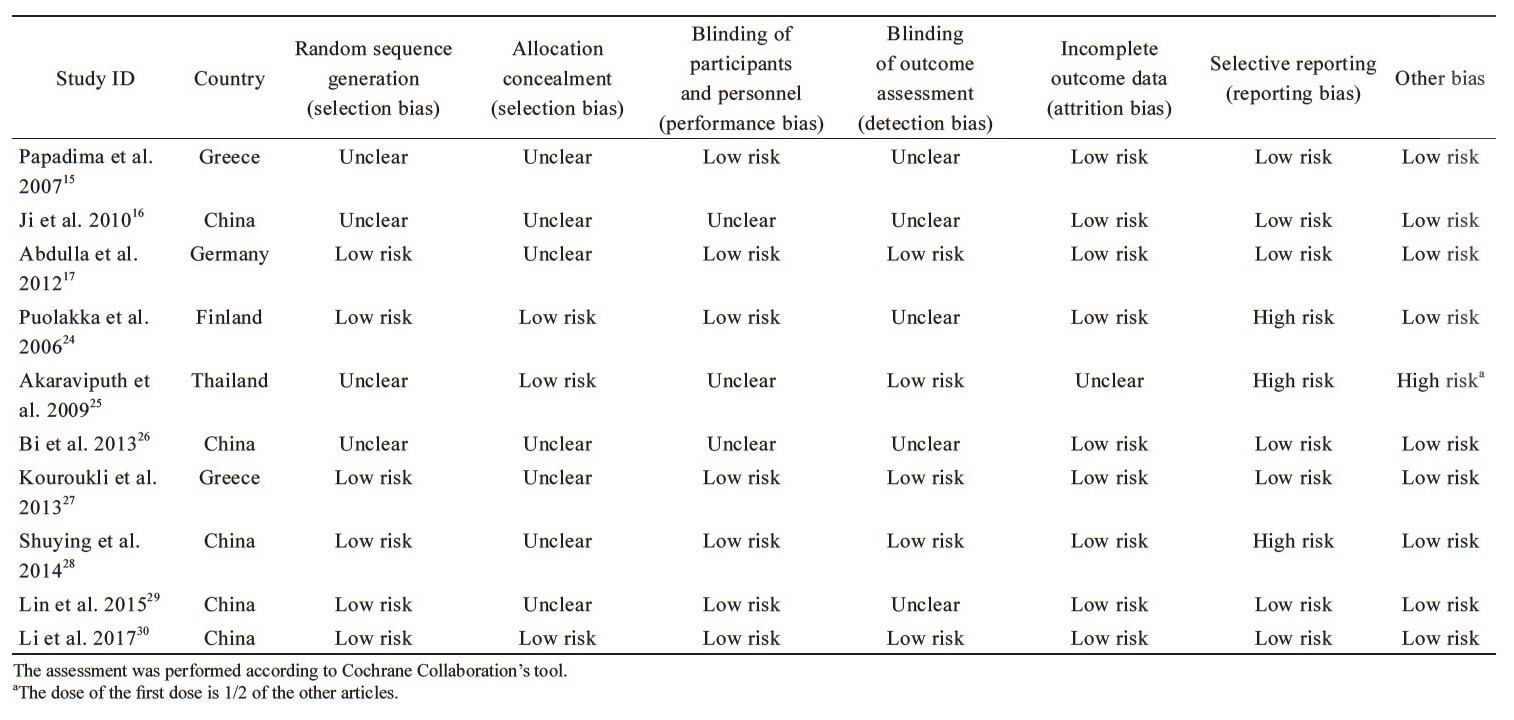
Download full-size image
Pain Scores
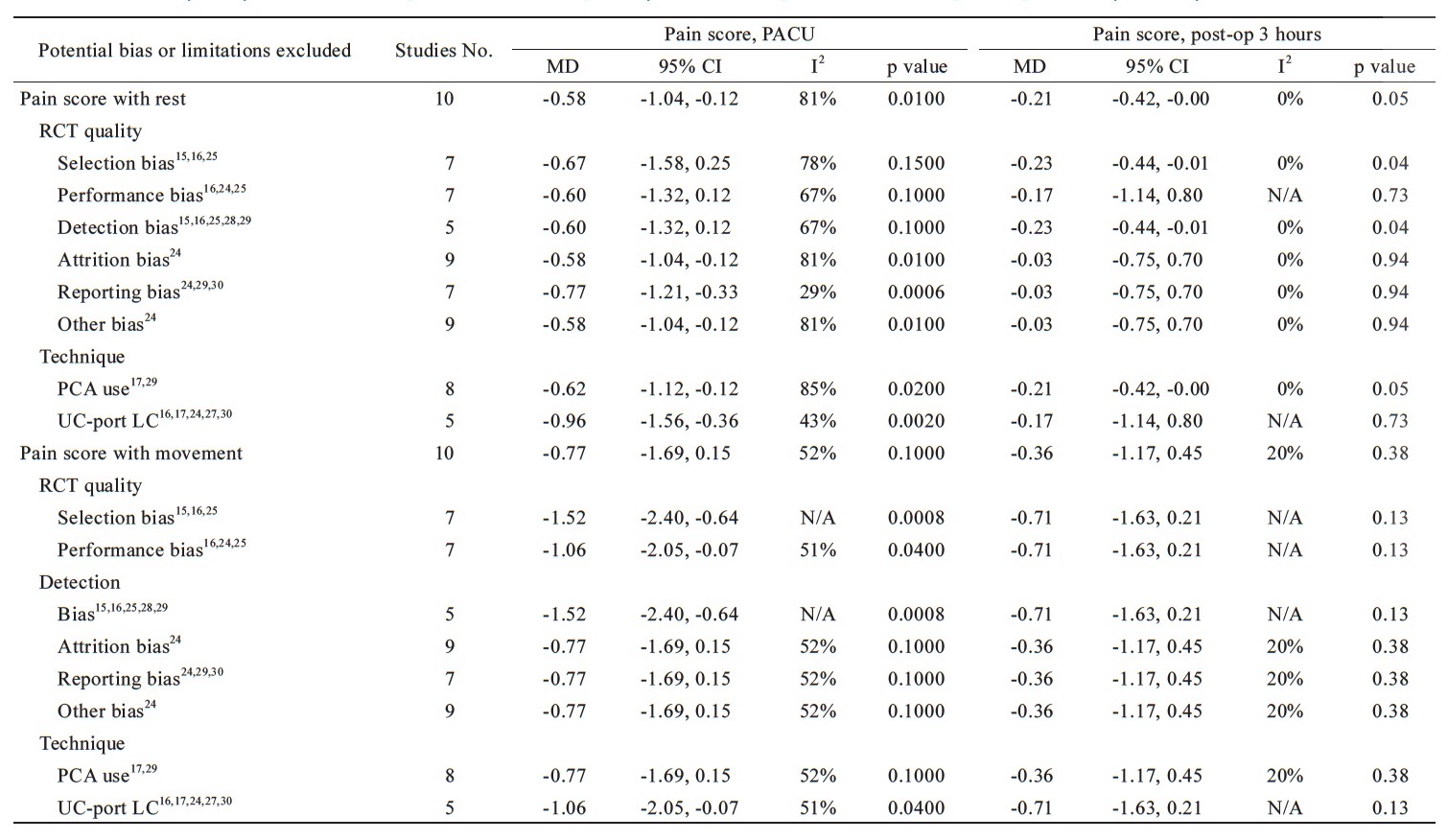
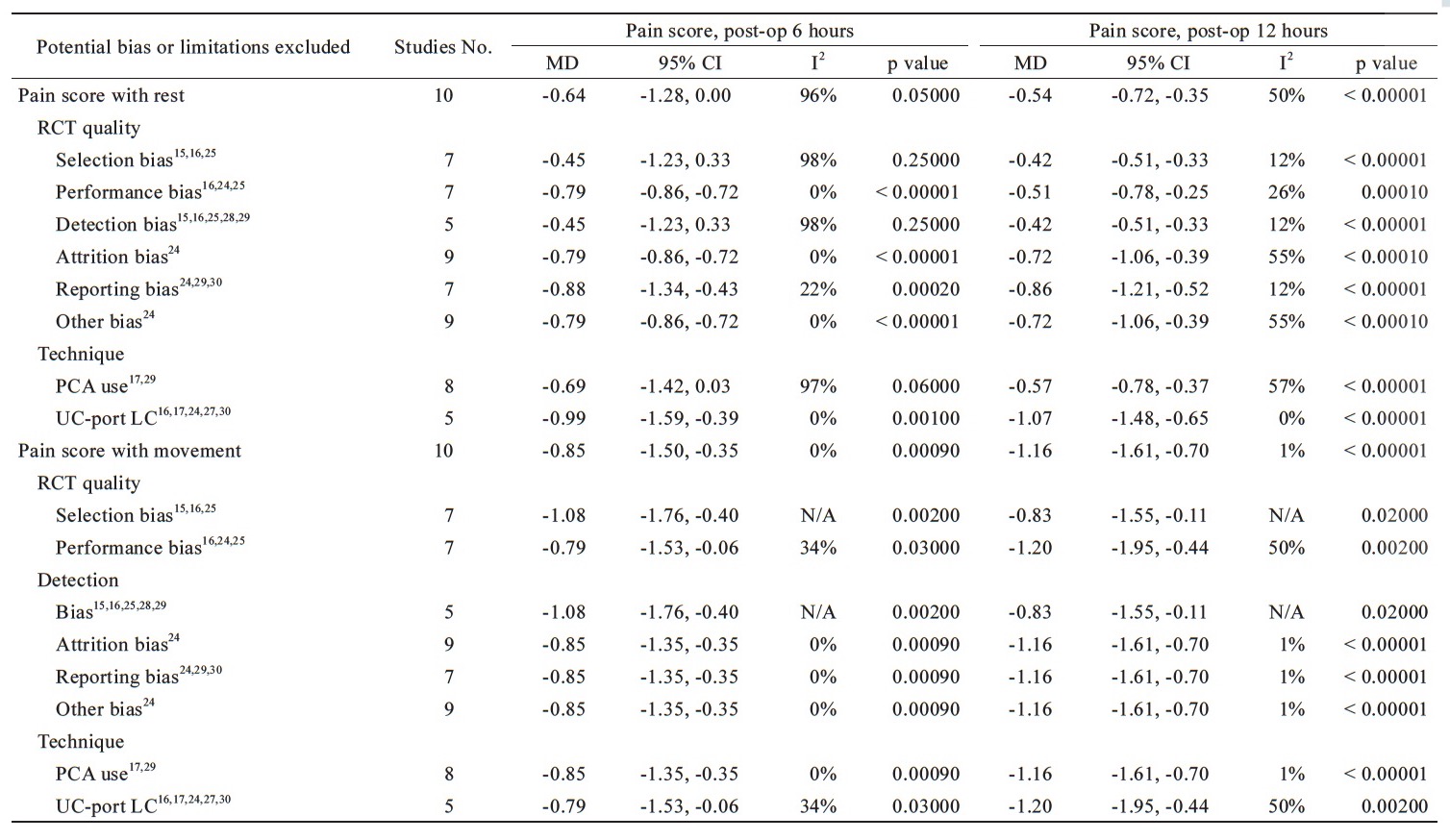
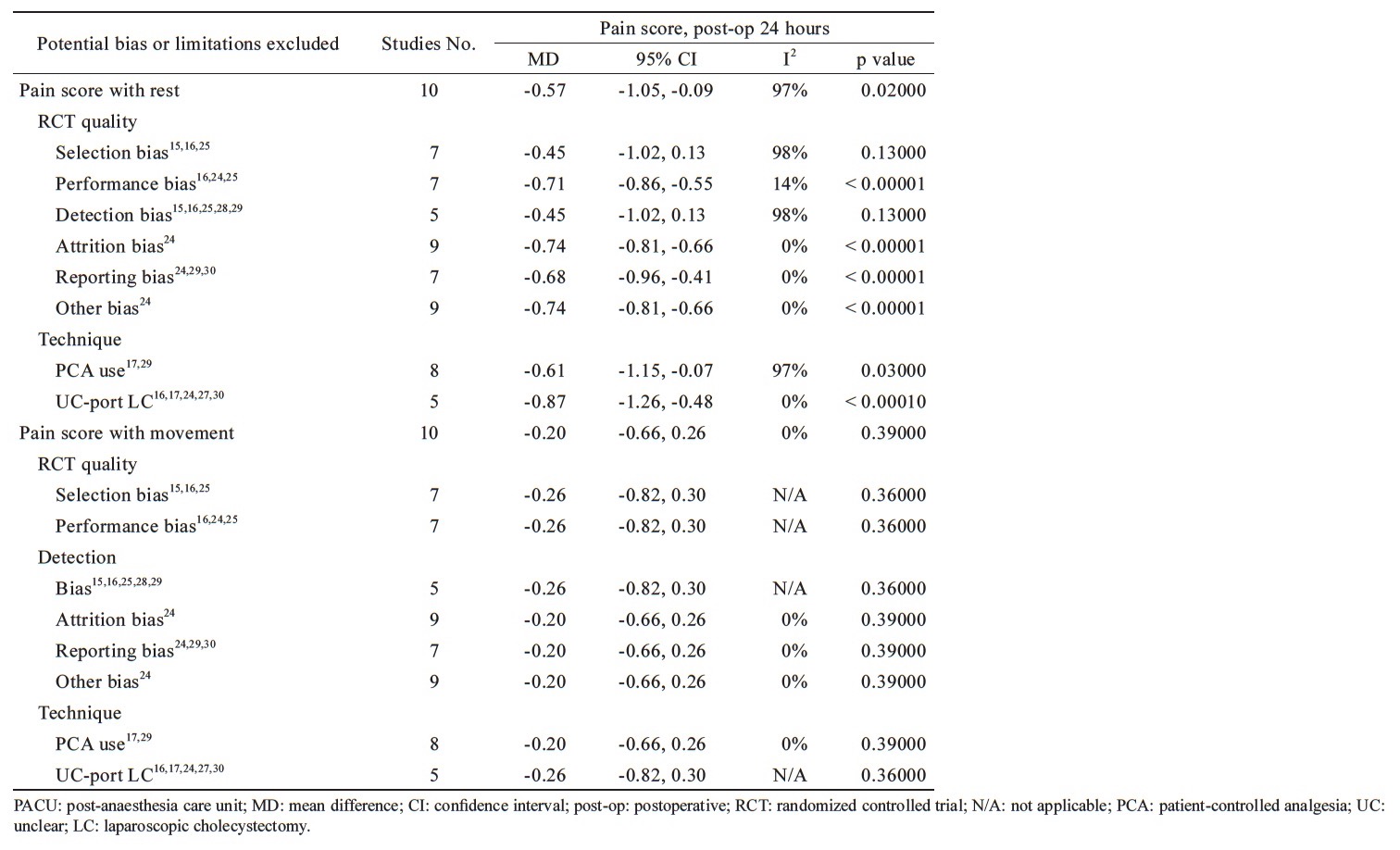
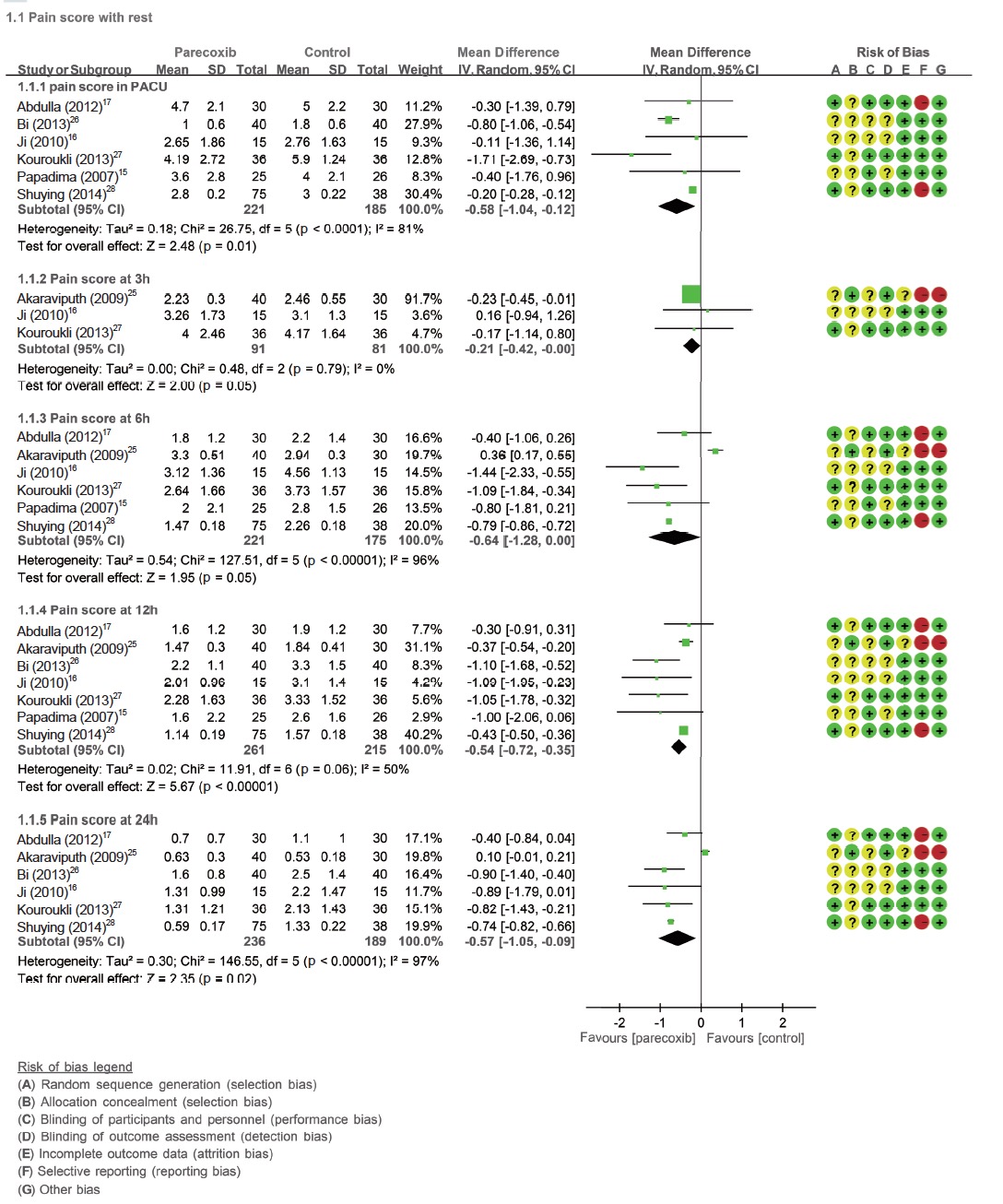
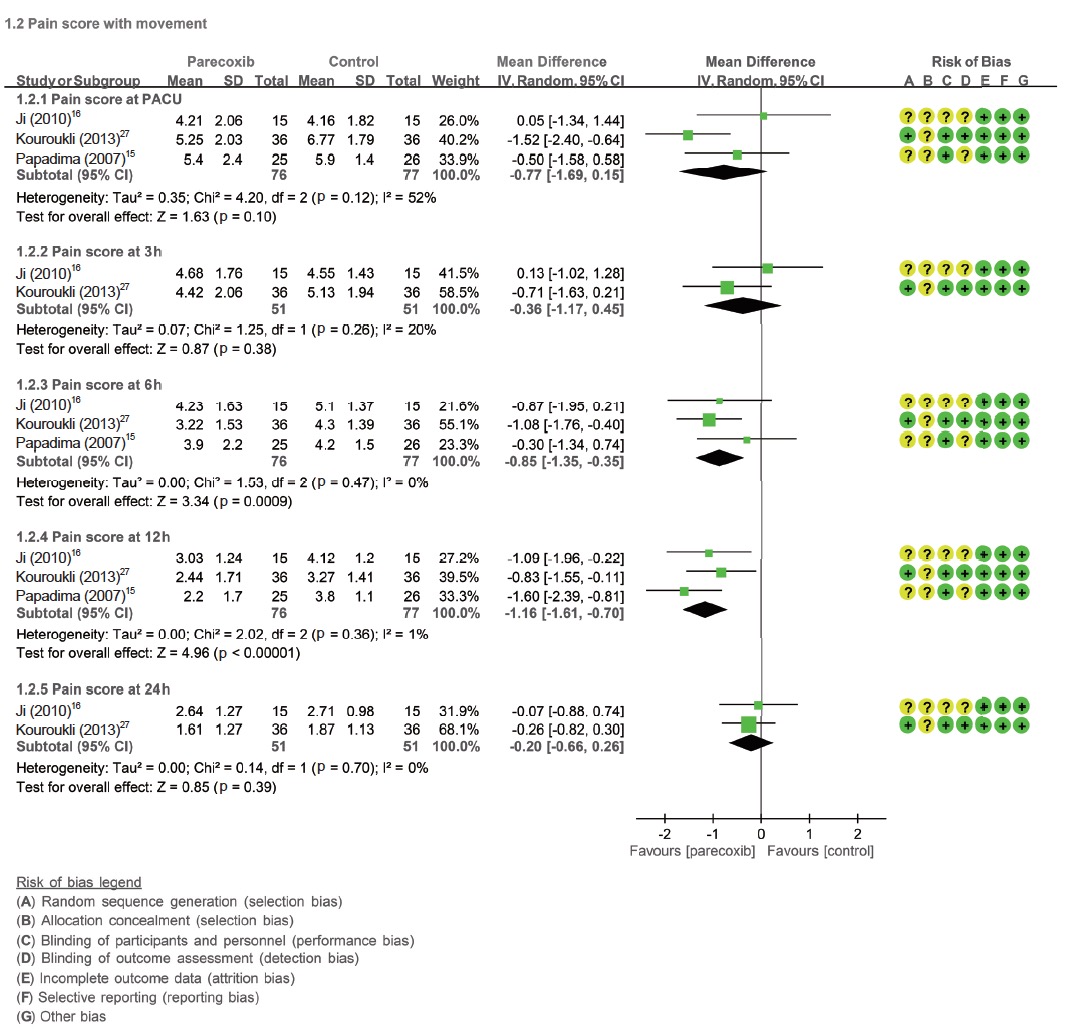
The pain score with rest at post-anaesthesia care unit (PACU) derived from six RCTs15-17,26-28 (n = 406) was significantly reduced in parecoxib groups when compared with control, with an MD of -0.58 (95% CI = -1.04 to -0.12, p = 0.01; I2 = 81%). The pain score with rest at postoperative 12 hours derived from seven RCTs15-17,25-28 and at 24 hours from six RCTs16,17,25-28 (n = 476 and 425) also were significantly reduced in parecoxib groups when compared with control, with MD of -0.54 (95% CI = -0.72 to -0.35, p < 0.00001; I2=50%) and MD of -0.57 (95% CI=-1.05 to -0.09, p=0.02; I2=97%), respectively. But the pain score with rest at postoperative three and six hours were not significantly reduced with MD of -0.21 (95% CI=-0.42 to 0.00, p=0.05; I2=0%) and MD of -0.64 (95% CI=-1.28 to 0.00, p=0.05; I2=96%), respectively (Fig. 2 and Suppl. Fig. 1). The pain socre with movement at PACU, postoperative three hours and 24 hours derived from three RCTs15,16,27 (n = 153), two RCTs16,27 (n = 102) and two RCTs16,27 (n = 102) were not significantly reduced in parecoxib groups when compared with control, with an MD of -0.77 (95% CI = -1.69 to 0.15, p = 0.1; I2 = 52%), MD of -0.36 (95% CI = -1.17 to 0.45, p = 0.38; I2 = 20%) and MD of -0.20 (95% CI = -0.66 to 0.26, p = 0.39; I2 = 0%), respectively. The pain score with movement at postoperative six hours and 12 hours derived form three RCTs15,16,27 (n = 153) were significantly reduced in parecoxib groups when compared with control, with MD of -0.85 (95% CI = -1.35 to -0.35, p = 0.0009; I2 = 0%) and MD of -1.16 (95% CI = -1.61 to -0.70, p < 0.00001; I2=1%), separately (Fig. 2 and Suppl. Fig. 2).
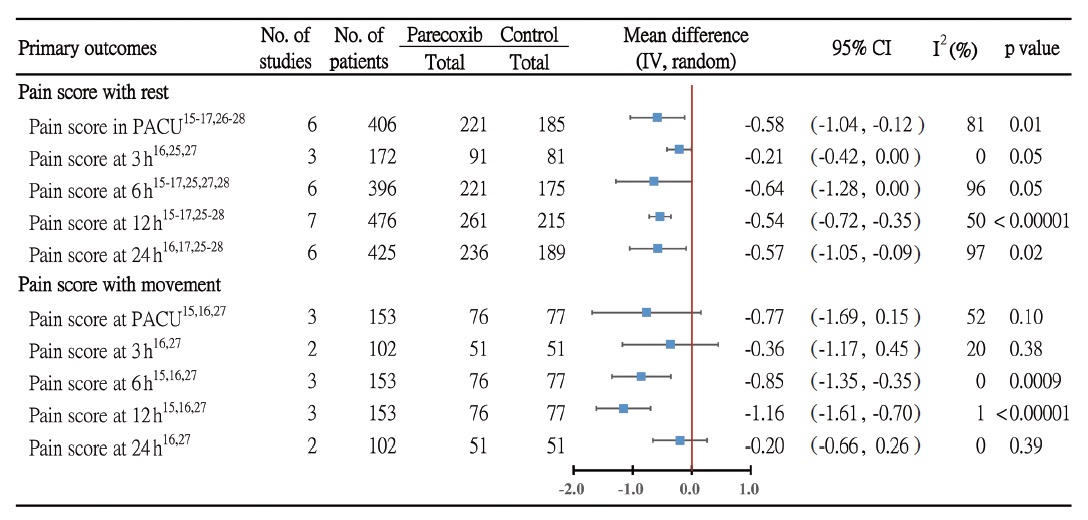
Download full-size image
Postoperative Morphine Consumption
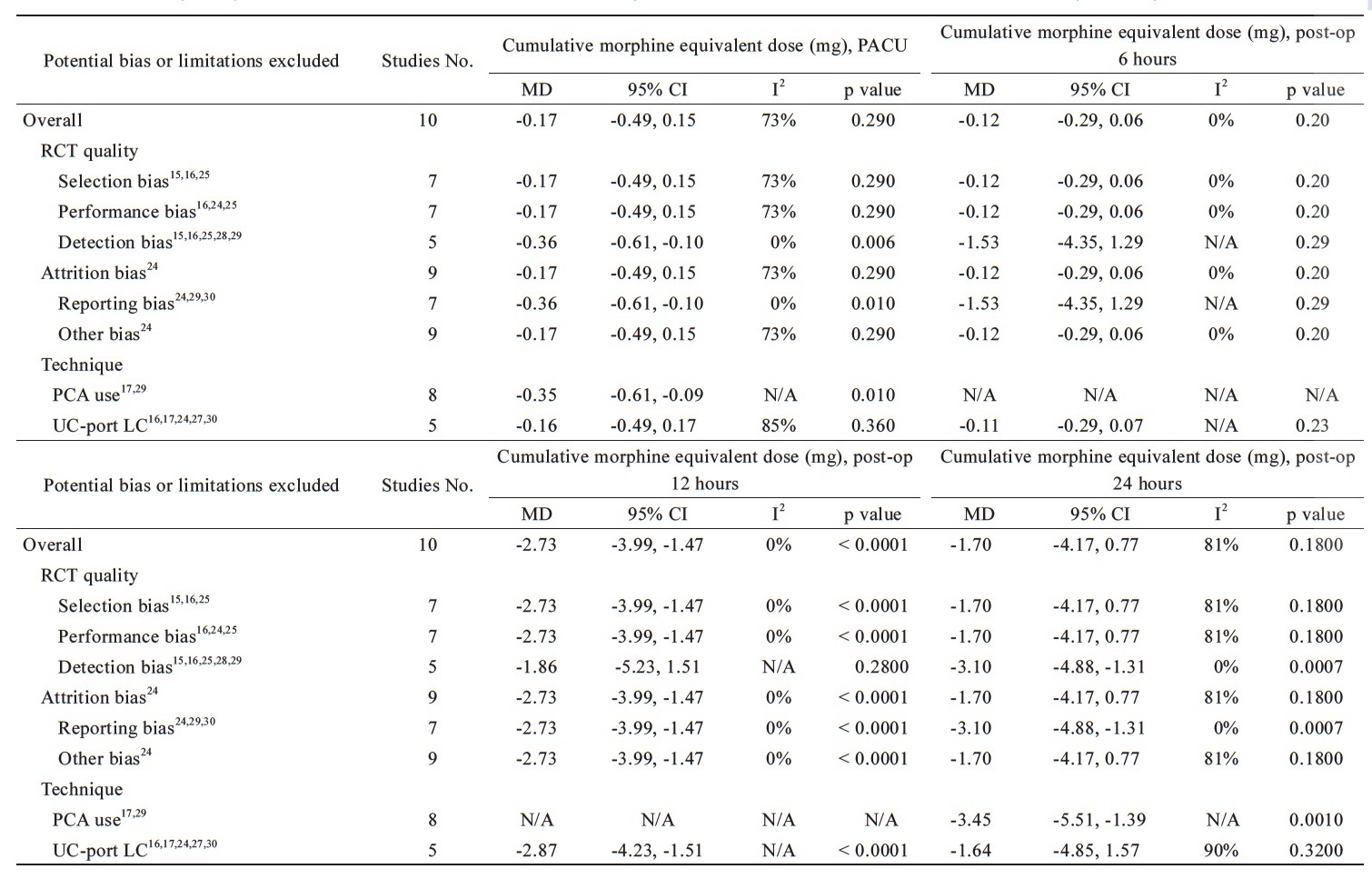
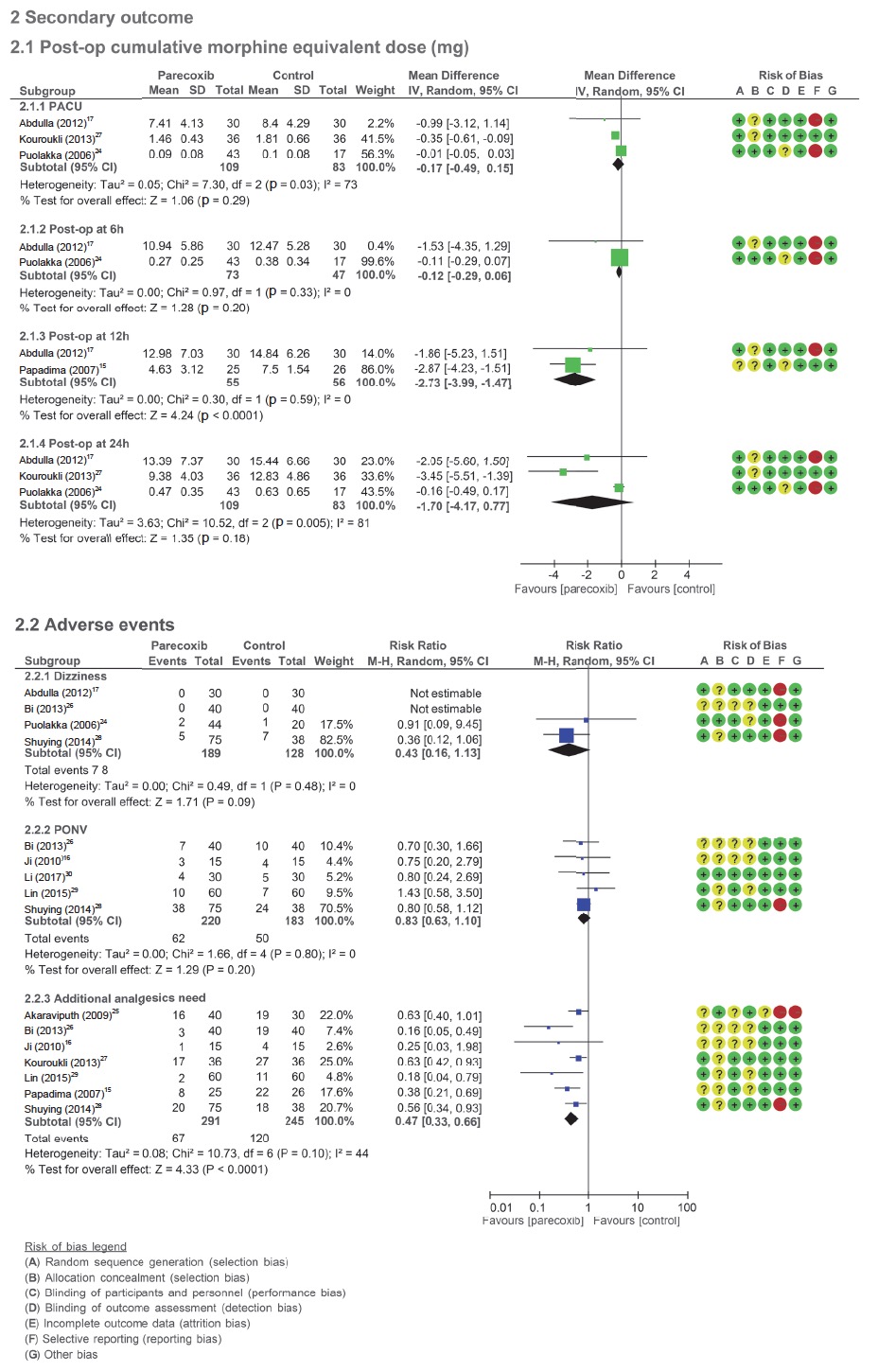
The postoperative cumulative morphine equivalent dose was reduced in the parecoxib groups at 12 hours15,17 after surgery with MD -2.7 mg (95% CI = -3.99 to -1.47, p < 0.0001; I2=0%), but the postoperative cumulative morphine equivalent dose was not reduced in the parecoxib groups at PACU17,24,27, six hours17,24 and 24 hours17,24,27 after surgery, the MD were -0.2 mg (95% CI = -0.49 to -0.15, p = 0.29; I2 = 73%), -0.1 mg (95% CI = -0.29 to 0.06, p = 0.2; I2 = 0%) and -1.7 mg (95% CI = -4.17 to 0.77, p = 0.18; I2 = 81%), separately (Fig. 3 and Suppl. Fig. 3).
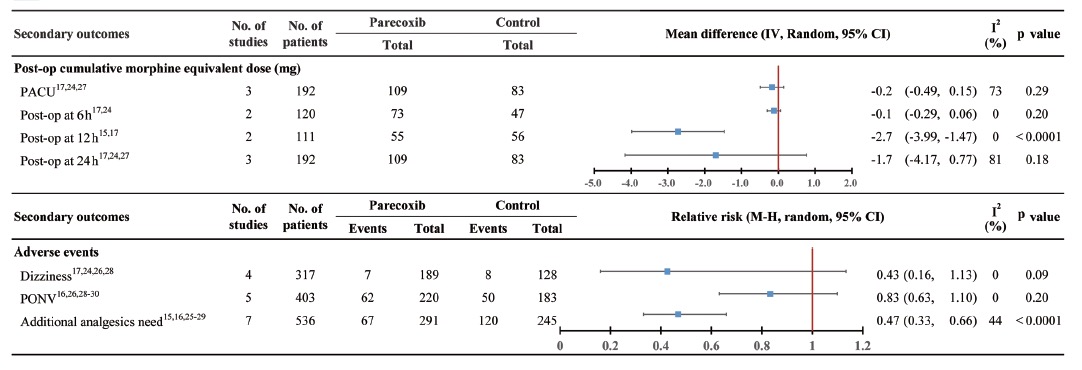
Download full-size image
Adverse Events
The adverse events incidence of dizzi-ness17,24,26,28 and postoperative nausea and vomiting (PONV)16,26,28,29,30 were not differences when parecoxib groups compared with the control group with RR 0.43 (95% CI = 0.16 to 1.13, p = 0.09; I2 = 0%) and 0.83 (95% CI = 0.63 to 1.10, p = 0.2; I2 = 0%), separately. The need for additional postopeative rescue analgesics15,16,25-29 was reduced in the parecoxib groups with RR = 0.47 (95% CI = 0.33 to 0.66, p < 0.0001; I2=44%) (Fig. 3 and Suppl. Fig. 3).
Publication Bias
The funnel plot showed the asymmetric distribution of studies about the pain score with rest at PACU, but Egger’s test was not significant (p = 0.26099; Fig. 4A). The “trim and fill” method results showed one necessary studies were missed. After filling the one with comprehensive analysis, the funnel plot showed improved symmetry (Fig. 4B). Under the random effects model, the MD and 95% CI for the combined studies is -0.58 (-1.04 to -0.12). Imputed standard error by “trim and fill” method is -0.63 (-1.07 to -0.19). The funnel plot showed the asymmetric distribution of studies about the pain score with movement at PACU, but Egger’s test was not significant (p = 0.18678; Fig. 5A). The “trim and fill” method results showed two necessary studies were missed. After filling the two with comprehensive analysis, the funnel plot showed improved symmetry (Fig. 5B). Under the random effects model, the MD and 95% CI for the combined studies is -0.77 (-1.69 to 0.15). Imputed standard error by “trim and fill” method is -1.52 (-2.56 to -0.48). Results indicated no evidence that publication bias or other known confounding variable the affected the outcomes, but other influencing factor interfering the intervention effect still needs to be considered.
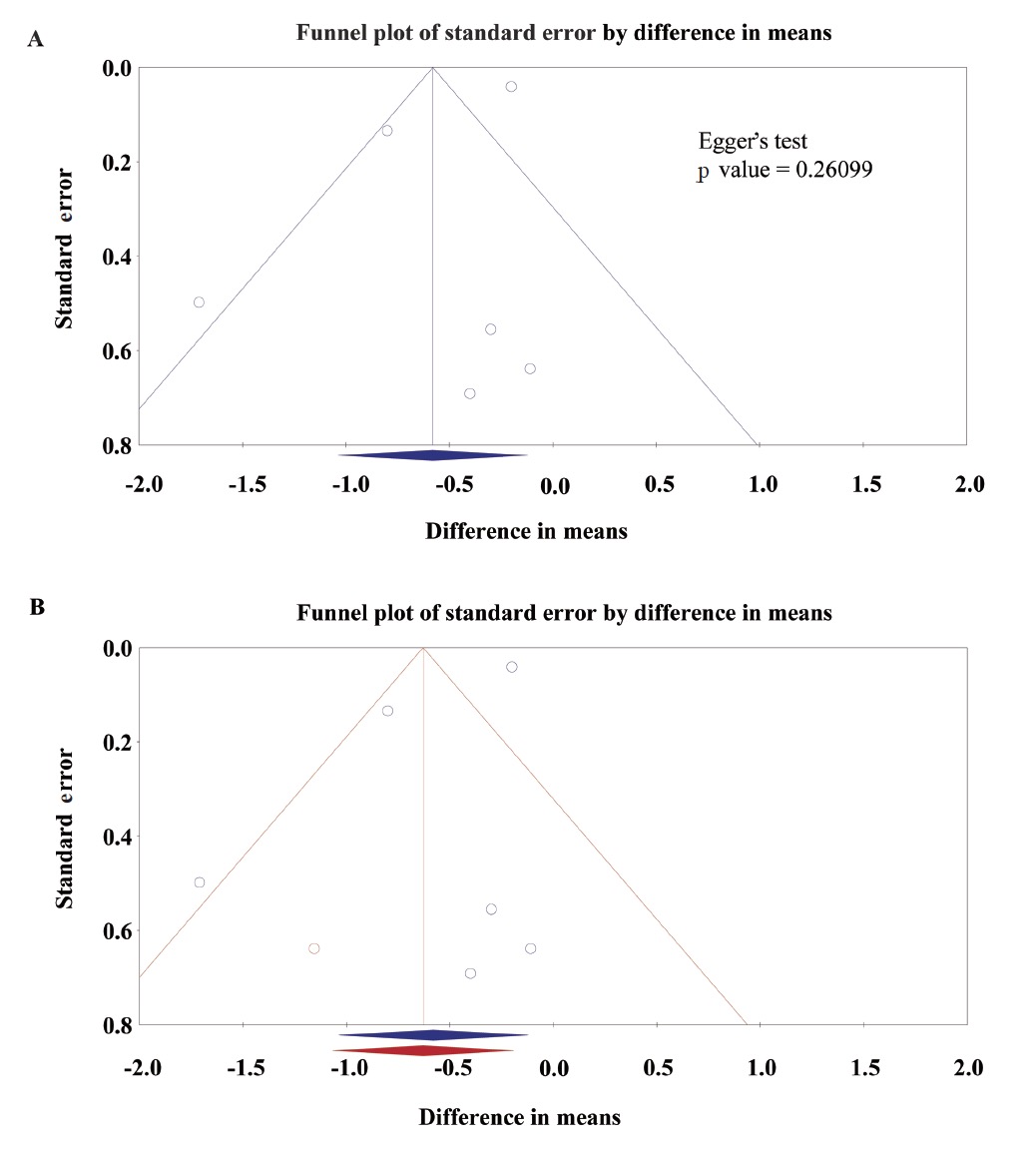
Download full-size image
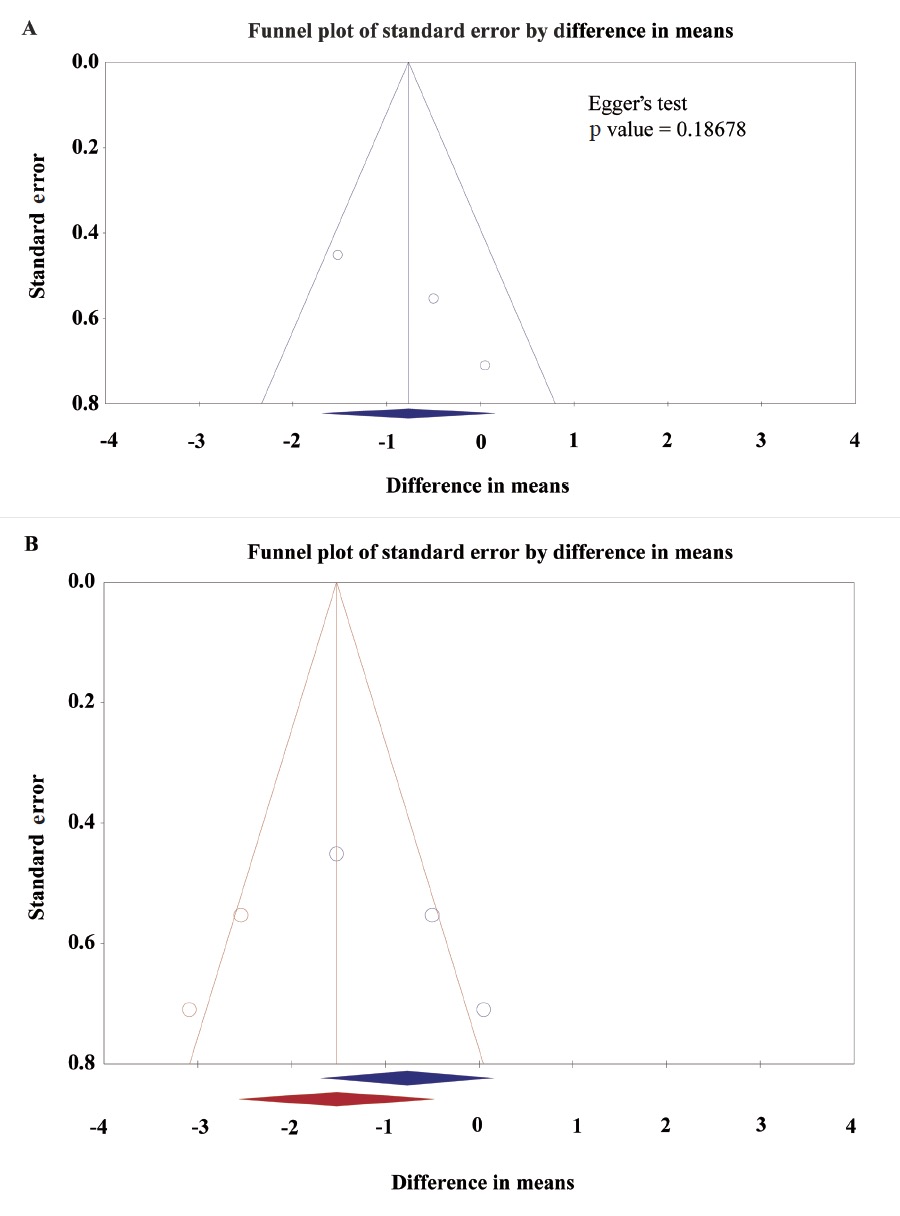
Download full-size image
Sensitivity Analyses
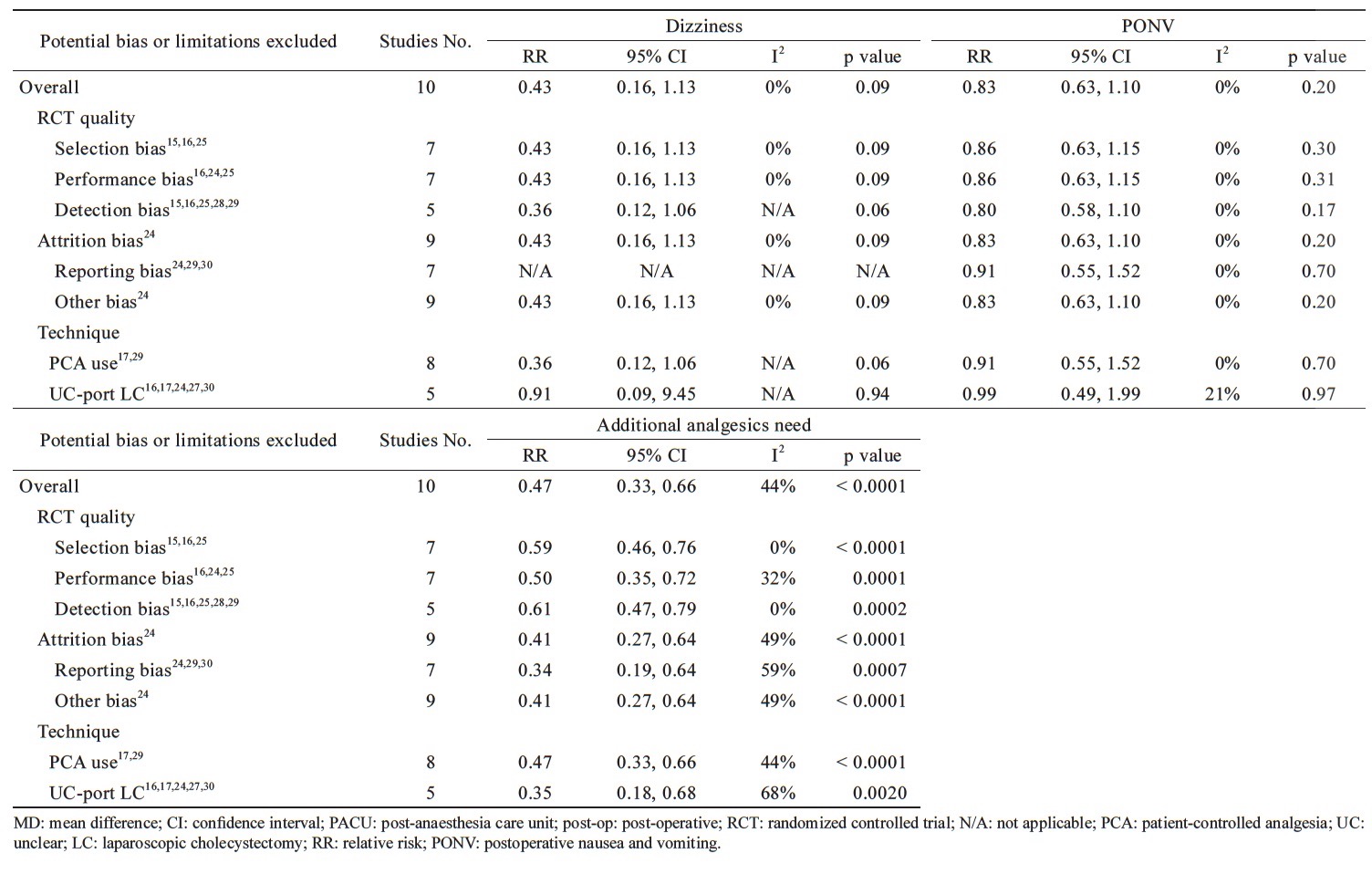
The sensitivity analysis of the potential bias is shown in Tables 3 and 4. There are 8 kinds of potential bias divided into two categories, RCT quality and various techniques applied, were considered. After exculding 3 trials with high risk of reporting bias,24,25,28 and 5 trials of unclear-port for LC,16,17,25,28,30 the MD of the pain score with rest at PACU was -0.77 (95% CI = -1.21 to -0.33, p = 0.0006; I2 = 29%) and -0.96 (95% CI = -1.56 to -0.36, p = 0.002; I2 = 43%), respectively. The significant decease of I2 showed these bias contributed the origin of heterogeneity without affecting the effect of parecoxib reducing the pain score with rest at PACU. In contrast, there was no difference between parecoxib and control groups in pain score with rest 3 hours after surgery when all kinds of bias being excluded.In analysis of pain score with rest at 6 hours postoperatively, the heterogeneity decreased and the pain relief effect of parecoxib became significant when excluding the performance bias16,25,26 (MD = -0.79, 95% CI = -0.86 to -0.72, p < 0.00001; I2=0%), attrition bias25 (MD = -0.79, 95% CI = -0.86 to -0.72, p < 0.00001; I2=0%), reporting bias24,25,28 (MD = -0.88, 95% CI = -1.34 to -0.43, p = 0.0002; I2 = 22%), other bias25 (MD = -0.79, 95% CI = -0.86 to -0.72, p < 0.00001; I2=0%) and unclear-port LC16,17,25,28,30 (MD = -0.99, 95% CI = -1.59 to -0.39, p = 0.001; I2 = 0%).
Download full-size image
Download full-size image
Download full-size image
Download full-size image
Download full-size image
In analysis of pain score with rest at 12 hours postoperatively, the heterogeneity decreased when excluding the bias of selection,15,16,26 performance,16,25,26 detection,15,16,24,26,29 reporting,24,25,28 and unclear-port LC16,17,25,28,30 without affecting the advantage of pain relief in parecoxib’s than controls with MD = -0.42, 95% CI = -0.51 to -0.33, p < 0.00001; I2=12%; MD=-0.51, 95% CI=-0.78 to -0.25, p=0.0001; I2=26%; MD=-0.42, 95% CI=-0.51 to -0.33, p < 0.00001; I2=12%; MD=-0.86, 95% CI=-1.21 to -0.52, p < 0.00001; I2=12%; MD=-1.07, 95% CI=-1.48 to -0.65, p < 0.00001; I2=0%, respectively. In analysis of pain score with rest at 24 hours postoperatively, parecoxib provided significant pain relief than controls when excluding the bias of performance, 16,25,26 attrition,25 reporting,24,25,28 others,25 and unclear-port LC16,17,25,28,30 with MD = -0.71, 95% CI = -0.86 to -0.55, p < 0.00001; I2=14%; MD=-0.74, 95% CI=-0.81 to -0.66, p < 0.0001; I2=0%; MD=-0.68, 95% CI=-0.96 to -0.41, p < 0.00001; I2=0%; MD=-0.74, 95% CI=-0.81 to -0.66, p < 0.00001; I2=0%; MD=-0.87, 95% CI=-1.26 to -0.48, p < 0.0001; I2=0%, respectively. In pain score with movement, the heterogeneity showed no significantly decreased when excluding the potential bias or limitations and no changes to the outcomes at PACU, postoperative three, six, 12, and 24 hours except the performance bias, and unclear-port LC for the pain score with movement at PACU (Table 3).
In analysis for cumulative morphine equivalent dose at PACU, heterogeneity decreased when excluding detection15,16,24,26,28 and reporting bias24,25,28 and parecoxib significantly reduced the cumulative morphine equivalent dose at PACU than control group with MD = -0.36 mg, 95% CI = -0.61 to -0.10, p = 0.006; I2 = 0% and MD = -0.36 mg, 95% CI = -0.61 to -0.10, p = 0.01. At six hours and 12 hours postoperatively, there was no significant effect in heterogeneity or cumulative morphine equivalent dose when potential bias or limitations being excluded. In analysis for cumulative morphine equivalent dose at 24 hours, the heterogeneity decreased and morphine consumption was significantly reduced after excluding detection15,16,24,26,29 and reporting bias24,25,28 with MD = -3.10 mg, 95% CI = -4.88 to -1.31, p = 0.0007; I2 = 0% and MD = -3.10 mg, 95% CI = -4.88 to -1.31, p = 0.0007; I2 = 0%, respectively (Table 4). In analysis for the adverse events, parecoxib did not increased risks for dizziness and PONV when excluding the potential bias or limitations. When excluding the bias of selection,15,16,26 performance,16,25,26 and detection,15,16,24,26,29 the heterogeneity decreased and parecoxib significantly reduced the incidence of additional analgesics need than control group with RR = 0.59, 95% CI = 0.46 to 0.76, p < 0.0001; I2=0%, RR=0.50, 95% CI=0.35 to 0.72, p=0.0001; I2=32% and RR=0.61, 95% CI=0.47 to 0.79, p=0.0002; I2=0%, respectively (Table 4).
Discussion
This meta-analysis being exclusively focused on pain control for elective LC surgery indicates that perioperative intravenous selective COX-2 inhibitor, parecoxib, could provide effective postoperative pain relief for patients receiving LC with significantly reduced consumption for postoperative morphine and additional analgesic supplement than the control groups without increasing adverse effects.
Pain and discomfort after patients receiving LC was mostly derived from the wound pain where dermatomes distributed around skin incision of puncture pores, vague peritoneal referred pain due to electrical cauterization around right upper quadrant of abdomen, and the shoulder discomfort coming from referred pain of diaphragm irritation due to carbon dioxide inflation during the laparoscopic surgery.3,8 Majority of contemporary pain management for postoperative pain relief counts on multimodal analgesia, including local injection of local anesthetics such as bupivacaine, intercostal nerve block, opioid analogues, acetaminophen, and selective or non-selective NSAID’s, for the postoperative pain relief for patients receiving LC.4-7,29 However, multiple prescription (multi-pharmacy) such as opioids, acetaminophen and various NSAID’s, would cause more concerns for adverse drug effects than pain relief per se.8 Searching for the simplest solution providing effective pain relief without potential adverse events for patients receiving LC who are expecting minimal length of hospital stay is more and more mandatory in clinical practice nowadays.
Mechanisms for the effectiveness of pain relief from preoperative parecoxib mainly constituted by both analgesic and systemic anti-inflammatory actions.31,32 This highly selective COX-2 inhibitor would be rapidly transformed to its active metabolite valdecoxib with potent analgesic effect and has been widely used to provide postoperative pain relief in various surgeries.10,11,33,34 As a sole analgesic for pain-relief, controversy in studies of parecoxib applied in minor-to-intermediate surgery such as LC.15-17 Different dosage regimen, timing of adopted drugs delivered, various control groups, and different time intervals for postoperative pain assessment complicated the results. This study showed that perioperative parecoxib provided statistically less pain score (better pain relief) at the time period of 12-hour after surgery measured at both resting and during movement in the PACU comparing the pain scale at time periods of 3- or 24-hour postoperatively. It implies that intravenous parecoxib 40 mg, as a single analgesic, could provide effective and better pain relief for patient receiving LC when it were delivered 6–8 hours before the surgery which was compatible with median time to onset of analgesic of parecoxib,35 rather than being given before the surgery start or postoperatively.
As to the postoperative morphine consumption and additional analgesic supplement, parecoxib could reduce central hyperalgesia and provide opioid-sparing effect for various surgeries in combination with other analgesic regimen.36 Our data validated that perioperative parecoxib 40 mg intravenously could significantly reduce the morphine consumption at the 12-hour interval postoperatively both at rest and movement. Different studies showed inconsistency between the attenuation of pain scale and opioid-sparing effect of parecoxib for the patients undergoing LC.17,24 Patient received parecoxib might experience significantly less postoperative morphine consumption without statistical difference in pain score reduction. Debatable outcomes might be derived from relatively small number of cases which was underpowered to verify the correlation between the effect of parecoxib to the pain scale and morphine consumption and further prospective study is needed. As an adjuvant agent, parecoxib was commonly applied with other pain-relief modalities such as PCA in orthopedic surgeries, total knee or hip arthroplasty were recommended.36 In order to amplify the analgesic effect, additional other COX-2 inhibitor supplement, such as valdecoxib in oral form, after LC surgery was also suggested.37,38 Less morphine, less morphine-related complications! In our study, there was also no statistical difference in the drug-related adverse effects such as dizziness, PONV when comparing the parecoxib and control groups receiving LC which was compatible with previous studies showed the efficacy of parecoxib in analgesia without overt side effects.33,34,36
Considering the heterogeneity among the studies included, the performance and detection bias contribute the sources of heterogeneity but the outcomes of less pain score and reduced morphine consumption under parecoxib was consistent when comparing other factors of bias in the analysis of sensitivity test.19,20 The analysis of publication bias for the rest or movement period after LC showed that after adjusting for missing studies, the point estimate of the overall effect size is approximately correct and coverage of the effect size CI is substantially improved. Although further studies expanding the case number is needed but it would not be a major influencing factor for the intervention effect.21-23
The strength of this meta-analysis includes several specific characteristics. First, comparing with other moderate-to-major surgeries,34,36 parecoxib was applied to be an adjuvant analgesic in addition to other major pain-relief protocol. This study focused on LC, a minor-to-moderate surgical procedure, parecoxib could be applied to be the sole analgesic and evaluating its potency and efficacy of pain-relief. Second, the control groups in the RCT recruited are relatively and exclusively simple, placebo or single analgesic such as injection of local anesthetics as controls, without other adjuvant or pain control regimen interfering the study design or outcomes evaluation, made the results relatively clear and conclusive. The third, the observation of postoperative period is relatively consistent among the studies recruited and without using other pain control modalities except for morphine and its equivalent analgesics made the comparison for outcomes being more standardized.
There are several limitation for the study. Various dosage regimen of parecoxib was only applied in one RCT. The best dosage regimen for the adopted drug, intravenous parecoxib, was still inclusive. Second, although nonparametric “trim and fill” method of accounting for publication bias was applied for analysis,22 further prospective studies to expand the case number is still needed to clarify its potency. Third, parecoxib was applied once perioperatively in most studies,15,16,24,28-30 instead of generalization for further dosage, such as second,17,24,25,28 third27 or more dosage26 postoperatively to maximize its analgesic effect as was routinely applied in other moderate-to-major surgeries. 34,36 However, the subsequent intravenous dosage of parecoxib might not be practical for the day-surgery or over-night surgery such as LC because most patients discharged soon after surgery which might limit the potential efficacy of subsequent intravenous parecoxib for postoperative pain-relief for LC. The fourth, this study focusing on patients undergoing elective LC surgery only, emergent surgery or other critical patients with complex medical conditions or abnormal liver functions, were not considered. The effectiveness or safety of parecoxib applying on these compromised patients receiving LC was not validated.
In conclusion, this meta-analysis showed that intravenous parecoxib given perioperatively, could effectively provide effective postoperative pain-relief, reduced morphine consumption and analgesic supplements without significant adverse effects for the non-emergent surgery of LC. This is applicable in the clinical practice suggesting parecoxib as a sole analgesic preoperatively for LC as an over-night surgery. Future studies remain needed for various dosage, different surgeries, or subsequent dosage of parecoxib postoperatively to further explore its potential analgesic effect.
Disclosure
The research received no specific grant from any funding agency in the public, commercial or not-forprofit sectors. Yu-Cih Lin, Chien-Yu Chen, Pi-Chu Lin, Yuan-Mei Liao, and Chuen-Chau Chang have no conflicts of interest or financial ties to disclose.
Author Contributions
Yu-Cih Lin and Chien-Yu Chen designed the study and searched the database. Yu-Cih Lin, Chien-Yu Chen, and Chuen-Chau Chang extracted, analyzed, and interpreted the data. Yu-Cih Lin wrote the first draft. Chuen-Chau Chang, Yuan-Mei Liao, and Pi-Chu Lin edited the manuscript. All authors contributed to subsequent versions and approved the final article. Chuen- Chau Chang is the corresponding author.
References
| 1 |
Steiner CA, Bass EB, Talamini MA, Pitt HA, Steinberg EP.
Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland.
N Eng J Med 1994;330:403–408.
|
| 2 |
Baron TH, Grimm IS, Swanstrom LL.
International approaches to gallbladder diseases.
N Engl J Med 2015;373:357–365.
|
| 3 |
Bisgaard T, Kehlet H, Rosenberg J.
Pain and convalescence after laparoscopic cholecystectomy.
Eur J Surg 2001;167:84–96.
|
| 4 |
Kehlet H, Dahl JB.
The value of “multimodal” or “balanced analgesia” in postoperative pain treatment.
Anesth Analg 1993;77:1048–1056.
|
| 5 |
Beaulieu P.
Non-opioid strategies for acute pain management.
Can J Anaesth 2007;54:481–485.
|
| 6 |
McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
Paracetamol and selective and non-selective non-steroid anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
Health Technol Assess 2010;14:1–153.
|
| 7 |
Elia N, Lysakowski C, Tramèr MR.
Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials.
Anesthesiology 2005;103:1296–1304.
|
| 8 | |
| 9 |
Daniels SE, Grossman EH, Kuss ME, Talwalker S, Hubbard RC.
A double-blind, randomized comparison of intramuscularly and intravenously administered parecoxib sodium versus ketorolac and placebo in a post-oral surgery pain model.
Clin Ther 2001;23:1018–1031.
|
| 10 |
Malan TP Jr, Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC.
Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty.
Anesthesiology 2003;98:950–956.
|
| 11 |
Ng A, Smith G, Davidson AC.
Analgesic effects of parecoxib following total abdominal hysterectomy.
Br J Anaesth 2003;90:746–749.
|
| 12 |
Ott E, Nussmeier NA, Duke PC, et al.
Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery.
J Thorac Cardiovasc Surg 2003;125:1481–1492.
|
| 13 |
Nussmeier NA, Whelton AA, Brown MT, et al.
Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery.
N Eng J Med 2005;352:1081–1091.
|
| 14 |
Joshi GP, Viscusi ER, Gan TJ, et al.
Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX-2 specific inhibitor.
Anesth Analg 2004;98:336–342.
|
| 15 |
Papadima A, Lagoudianakis EE, Antonakis PT, et al.
Parecoxib vs. lornoxicam in the treatment of postoperative pain after laparoscopic cholecystectomy: a prospective randomized placebo-controlled trial.
Eur J Anaesthesiol 2007;24:154–158.
|
| 16 |
Ji FH, Jin X, Yang JP, Zan LL.
Analgesic effect of parecoxib and flurbiprofen axetil for patients undergoing laparoscopic cholecystectomy and their influences on platelet aggregation.
Chin Med J (Engl) 2010;123:1607–1609.
|
| 17 |
Abdulla S, Eckhardt R, Netter U, Abdulla W.
A randomized, double-blind, controlled trial on non-opioid analgesics and opioid consumption for postoperative pain relief after laparoscopic cholecystectomy.
Acta Anaesthesiol Belg 2012;63:43–50.
|
| 18 |
Moher D, Liberati A, Tetzlaff J, Altman DG.
Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.
BMJ 2009;339:b2535.
|
| 19 |
Higgins JPT, Altman DG.
Assessing risk of bias in included studies.
In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd; 2008:187–241.
|
| 20 |
DerSimonian R, Laird N.
Meta-analysis in clinical trials.
Control Clin Trials 1986;7:177–188.
|
| 21 |
Egger M, Davey Smith G, Schneider M, Minder C.
Bias in meta-analysis detected by a simple, graphical test.
BMJ 1997;315:629–634.
|
| 22 |
Duval S, Tweedie R.
A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis.
J Am Stat Assoc 2000;95:89–98.
|
| 23 |
Taylor S, Tweedie R.
Practical estimates of the effect of publication bias in meta-analysis.
Australas Epidemiol 1998;5:14–17.
|
| 24 |
Puolakka PAE, Puura AIE, Pirhonen RA, et al.
Lack of analgesic effect of parecoxib following laparoscopic cholecystectomy.
Acta Anaesthesiol Scand 2006;50:1027–1032.
|
| 25 |
Akaraviputh T, Leelouhapong C, Lohsiriwat V, Aroonpruksakul S.
Efficacy of perioperative parecoxib injection on postoperative pain relief after laparoscopic cholecystectomy: a prospective, randomized study.
World J Gastroenterol 2009;15:2005–2008.
|
| 26 |
Bi RB, Ji L, Wang XK.
Effect analysis of multimode analgesia of parecoxib sodium during laparoscopic cholecystectomy.
[In Chinese, English abstract]. Zhonghua Yi Xue Za Zhi 2013;93:2727–2729.
|
| 27 |
Kouroukli I, Zompolas V, Tsekoura V, Papazoglou I, Louizos A, Panaretou V.
Comparison between lornoxicam quick-release and parecoxib for post-operative analgesia after laparoscopic cholecystectomy: a prospective randomized, placebo-controlled trial.
J Anaesthesiol Clin Pharmacol 2013;29:485–490.
|
| 28 |
Shuying L, Xiao W, Peng L, Tao Z, Ziying L, Liang Z.
Preoperative intravenous parecoxib reduces length of stay on ambulatory laparoscopic cholecystectomy.
Int J Surg 2014;12:464–468.
|
| 29 |
Lin S, Hua J, Xu B, et al.
Comparison of bupivacaine and parecoxib for postoperative pain relief after laparoscopic cholecystectomy: a randomized controlled trial.
Int J Clin Exp Med 2015;8:13824–13829.
|
| 30 |
Li X, Liu J, Zhou M, Zhou C.
Parecoxib sodium pretreatment reduces myoclonus after etomidate: a prospective, double-blind, randomized clinical trial.
Int J Clin Pharmacol Ther 2017;55:601–605.
|
| 31 |
Wu Q, Purusram G, Wang H, et al.
The efficacy of parecoxib on systemic inflammatory response associated with cardiopulmonary bypass during cardiac surgery.
Br J Clin Pharmacol 2013;75:769-778.
|
| 32 |
Tegeder I, Pfeilschifter J, Geisslinger G.
Cyclooxygenase-independent actions of cyclooxygenase inhibitors.
FASEB J 2001;15:2057–2072.
|
| 33 |
Malan TP Jr, Gordon S, Hubbard R, Snabes M.
The cyclooxygenase-2-specific inhibitor parecoxib sodium is as effective as 12 mg of morphine administered intramuscularly for treating pain after gynecologic laparotomy surgery.
Anesth Analg 2005;100:454–460.
|
| 34 |
Nussmeier NA, Whelton AA, Brown MT, et al.
Safety and efficacy of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib after noncardiac surgery.
Anesthesiology 2006;104:518–526.
|
| 35 |
Cheer SM, Goa KL.
Parecoxib (parecoxib sodium).
Drugs 2001;61:1131–1141.
|
| 36 |
Wei W, Zhao T, Li Y.
Efficacy and safety of parecoxib sodium for acute postoperative pain: a meta-analysis.
Exp Ther Med 2013;6:525–531.
|
| 37 |
Gan TJ, Joshi GP, Zhao S, Hanna DB, Cheung RY, Chen C.
Presurgical intravenous parecoxib sodium and follow-up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid-related adverse effects.
Acta Anaesthesiol Scand 2004;48:1194–1207.
|
| 38 |
Gan TJ, Joshi GP, Viscusi E, et al.
Preoperative parenteral parecoxib and follow-up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery.
Anesth Analg 2004;98:1665–1673.
|
Appendix
Download full-size image
Download full-size image
Download full-size image