Abstract
Objective
After primary total knee/hip replacement (TKR or THR respectively) a prosthetic joint infection (PJI) could develop. Hypothermia could raise the risk of infection. Heating by forced-air can disrupt laminar airfl ow in the operation room (OR), potentially raising the risk of infection. We aimed to study non-inferiority of an active self-heating blanket (SHB) compared to a forced-air blanket (FAB) in preventing hypothermia.
Methods
A randomized controlled non-inferiority trial (N = 86 patients) was performed comparing a SHB versus a FAB in elective primary TKR/THR patients. Primary outcome was lowest measured temperature during surgery. Secondary outcomes were patients’ core temperature before, during, and after surgery, thermal comfort visual analogue score (VAS) and complications during hospitalization.
Results
Lowest measured temperature was 35.9°C (± 0.6) in SHB and 36.1°C (± 0.5) in FAB group (p = 0.05). No signifi cant correlation was found with duration of surgery or temperature of the OR. No signifi cant difference in core temperature was found before surgery (SHB = 36.8°C [± 0.4], FAB = 36.8°C [± 0.5], p = 0.49), after induction of anaesthesia (SHB = 36.6°C [± 0.5], FAB = 36.7°C [± 0.5], p = 0.22) nor as a mean during surgery (SHB = 35.8°C [± 1.6], FAB = 36.0°C [± 1.3], p = 0.68). SHB patients were “colder” at the recovery bay, 35.8°C (± 0.6) compared to FAB patients, 36.1°C (± 0.5) (p = 0.04). Mean VAS thermal comfort was 53.3 (± 15.7) in SHB and 52.9 (± 12.3) in FAB patients. No difference in complication rate was found.
Conclusion
In this study neither kind of the warming blanket prevented perioperative hypothermia. Although a difference of 0.2°C was found between both groups at the end of TKR/THR surgery, this is most probably not clinically relevant. Complication rate in both groups was the same.
Keywords
inadvert perioperative hypothermia (IPH), Bair HuggerTM, BARRIER EasyWarmTM, arthroplasty
Introduction
Most general anaesthetics impair thermoregulatory responses resulting in inadvertent perioperative hyporthermia (IPH).1 IPH, defined as a body temperature between 34.0°C and 36.0°C, during primary total knee or hip replacement surgery (TKR or THR respectively) is associated with adverse events.2 Studies showed that IPH might result in more postoperative discomfort, prolonged length of hospital stay, and a higher risk of surgical site infection.3-5 This is why warming of joint replacement patients has become routine practice. Several strategies can be used to warm patients during surgery; two available techniques are active warming by forced-air devices or warming using self-warming blankets.6,7
Clean laminar airflow in operating rooms (ORs) might reduce the risk of infection in TKR or THR surgery.8 This downward directed airflow is shown to be disrupted by forced-air warming devices when hot air moves upwards against this downward air current.8,9 Furthermore, this upwards directed air current could theoretically induce prosthetic joint infection (PJI) by creating air currents with a downward directed flow on the operating field.10
In an effort to further reduce the risk of developing PJI, we hypothesised that using a self-warming blanket would keep the core temperature of the patient at the end of surgery at the same level as the forced air devices, but with the advantage that no air currents were present. Therefore, we aimed to study the non-inferiority of the self-warming blanket compared to the, more frequently used, forced-air warming.
Methods
This prospective, randomized controlled, single-center non-inferiority trial was approved by the Medical Ethics Committee (METC ZWH, No.17-049) and the board of directors of the Haga Hospital. The trial was registered in the Dutch Trial Registry(NTR6495).
Participants
Inclusion took place between June and August 2017. All consecutive patients who were planned for primary TKR or THR surgery, 18 years and over of age and able to speak and understand the Dutch language were considered eligible and were asked to participate in the study. They were included after signing informed consent. Patients with severe peripheral arterial disease were excluded from the study. All surgeries were performed in one general training hospital in the Netherlands. In this hospital, there are dedicated hip and knee surgeons and approximately 900 THR and 350 TKR are performed each year. A total of 80 patients were included, according to the sample size calculation 33 patients in each group were needed but we added seven extra patients to compensate for withdrawal and missing data (non-inferiority design, power = 90%, standard deviation [SD] = 0.6°C)
Intervention
Participants were randomized into one of two treatment groups:
(1) Forced-air warming using the Bair Hugger™ device (3M Co., St. Paul, MN, USA), by machine generated heated air that is inflated into airpockets of the blanket. (2) Self-warming blanket BARRIER EasyWarm™ (Mölnlycke Health Care AB, Götenborg, Sweden), warming by an exothermic chemical reaction (oxidation of iron) in each of the 12 compartments within the blanket. The compartments consist of activated carbon, iron, water, salt, clay, and supportive chemicals.11At the ward all participants received the self-warming blanket to pre-heat before going to the OR (which is standard protocol of care at our institution). At the anaesthesiology bay patients received the SpotOn™ (3M Co.) thermometer.12 This is a non-invasive device continuously measuring and registering core body temperature.12 All data were directly saved in the electronic patient-care system. At the end of the surgery, tympanic temperature was recorded as well. Patients in both groups were operated on according to the standard protocol for TKR or THR. The surgeries were performed at the same location, but in four different operating theatres. In case of THR, the patient was supine, and the direct anterior approach was used in all cases. In case of TKR, patients were also in supine position and the median incision, and medial parapatellar approach was used in all cases. Both warming systems were applied on the upper part of the body of the patient in a way that most of the skin was covered by the blanket. There were no warmed intravenous fluids used because it could bias the outcome. Temperature of the OR during all procedures was recorded continuously. Upon return of the patient at the postoperative recovery bay a visual analogue scale (VAS) regarding temperature comfort experience was recorded. The scale on this VAS ranged from extremely cold (0) to extremely hot (100).
Outcomes
Primary outcome was the lowest temperature measured during surgery. Secondary outcomes measures were core temperature preoperatively at the holding, after induction of anaesthesia, intraoperatively and postoperatively at the recovery ward. Also, tympanic temperature at the end of surgery, the total number of measurements < 36.0°C, thermal comfort VAS and complications during hospitalisation were recorded. Complications were defined as adverse events occurred during the study, regardless whether they were related to the use of the Bair Hugger™ or BARRIER EasyWarm™ or not.
Randomization
Allocation of treatment sequence was generated by a computer using Castor EDC data management software (Castor EDC, Amsterdam, the Netherlands) and was concealed until the very last moment. Variable block randomization was used with block sizes of 2, 3, or 4.
Before entering the OR center, the bed of the patient was tagged with the allocated treatment by one of the investigators who did not assess outcomes. Blinding during surgery was not possible due to the obvious differences between the two warming systems. Investigators assessing outcomes were blinded for treatment allocation.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA). To calculate sample size, a power analysis for equivalence (unpaired test) was performed. Based on Brandt et al.13 in 2010, lower and upper equivalence bounds were ± 0.5°C, with a SD of 0.6°C. To achieve a power of 90% to detect equivalence within the equivalence bounds of ± 0.5°C, a total sample size of 43 patients per group (86 patients) was estimated, including loss of follow-up. If we measured a significant difference in infection rate between the two methods, we would need thousands of patients.
The primary outcome was analysed by a twoone sided test (TOST), a test of equivalence that is based on the classical t-test used to test the hypothesis of equality between two means, as well as an independent sample t-test.14
Demographic variables, secondary outcomes regarding to core temperatures and thermal comfort (VAS) were calculated with independent samples t-test. We checked whether OR temperature and duration of surgery were confounders for the outcomes with a linear regression analysis. Dichotomous variables were calculated by chi-squared test. We also calculated the interclass correlation (ICC) between the SpotOn™ core thermometer and tympanic temperature. Results when normally distributed are expressed in mean (± SD) or 95% confidence intervals (CIs). Differences were considered statistically significant at p < 0.05.
Results
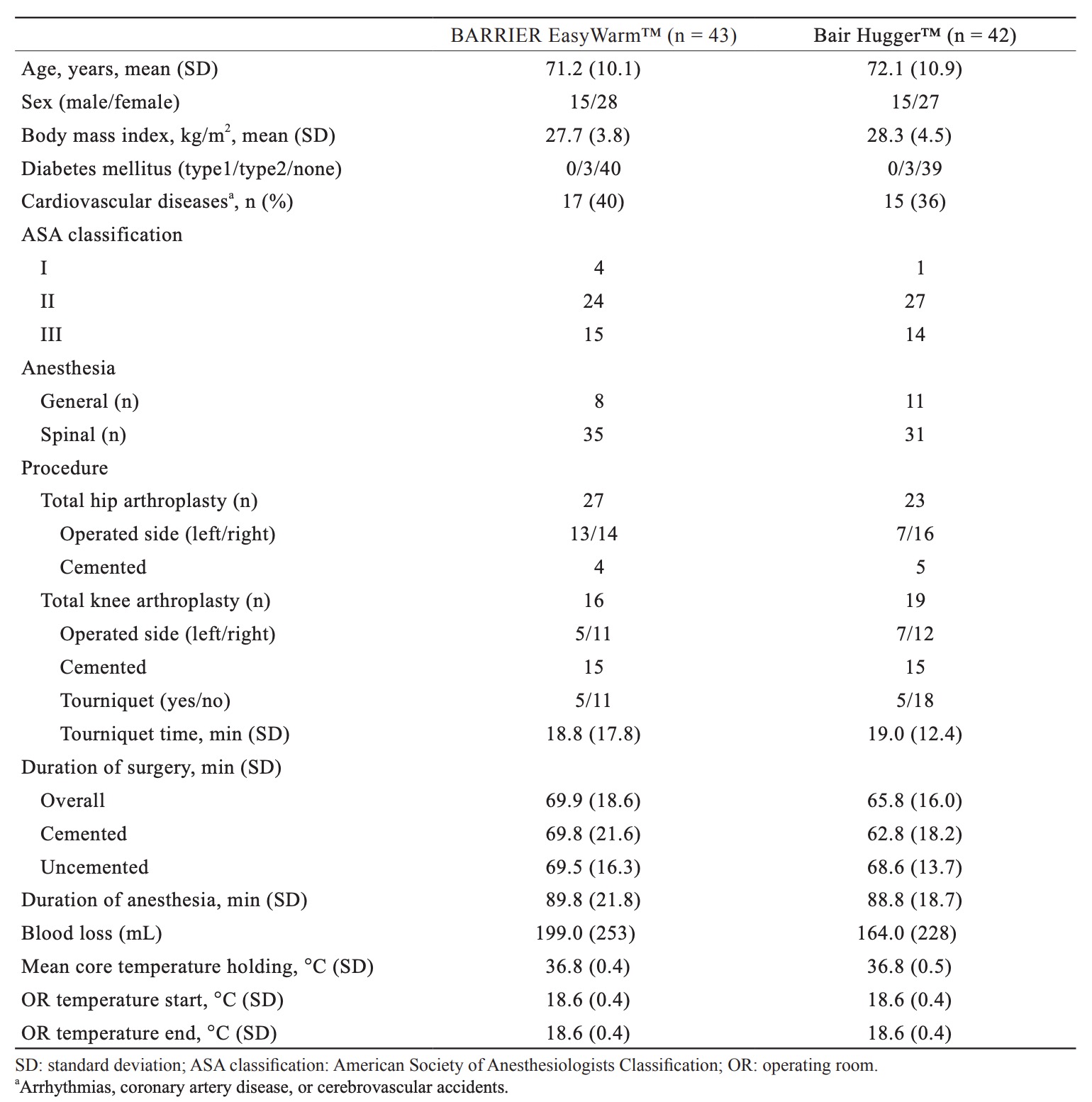
From 90 consecutive patients 86 were randomized to receive one of the two warming systems (Fig. 1). All patients were treated according to allocation. Table 1 shows baseline characteristics per treatment group. Groups were comparable in terms of demographic and clinical characteristics. The mean OR temperature at the start and end of the operation, the duration of surgery, and the amount of blood loss did not differ between the two groups.
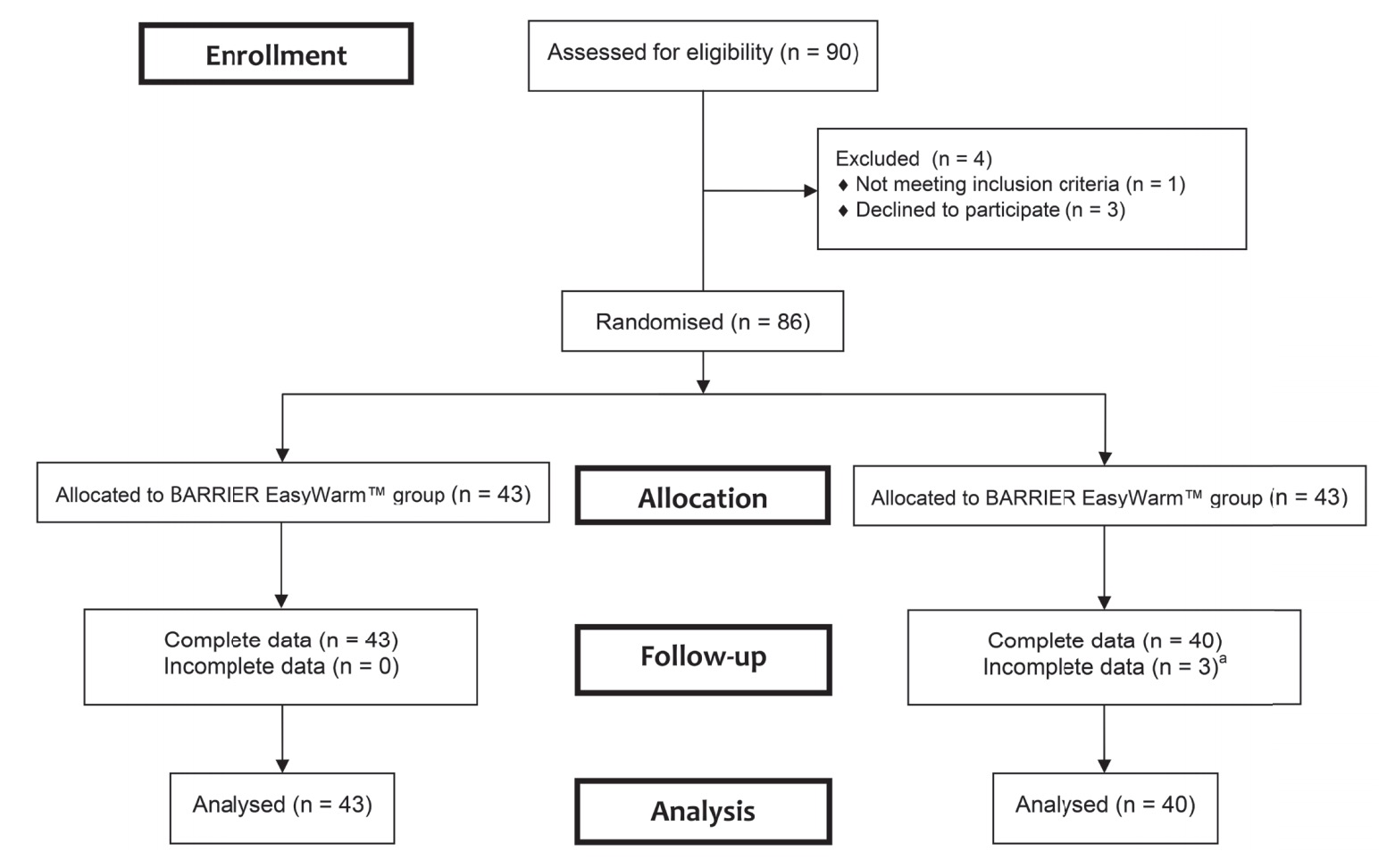
Download full-size image
a Two patients had no data due to mechanical failure of the temperature monitoring system; one patient had no data because the surgery was cancelled after she was included.
Download full-size image
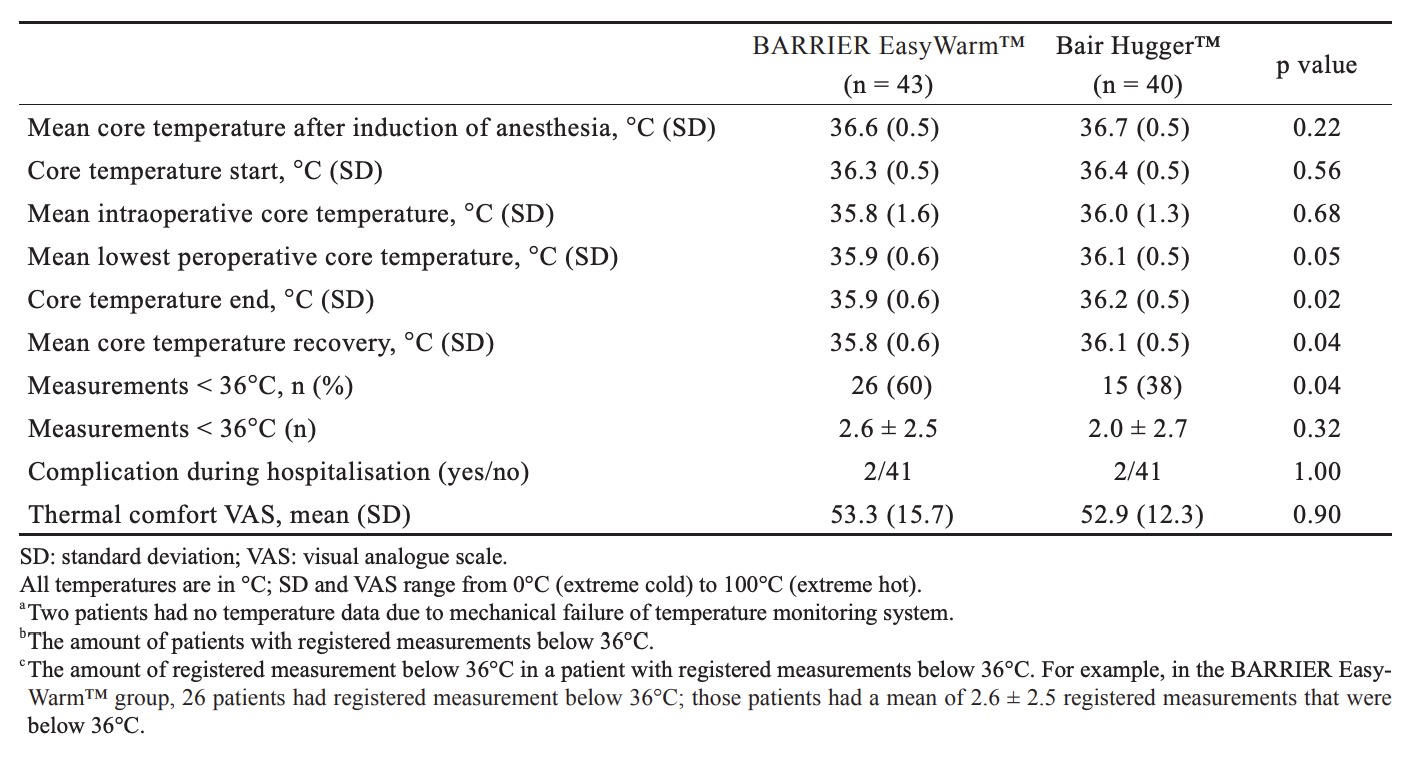
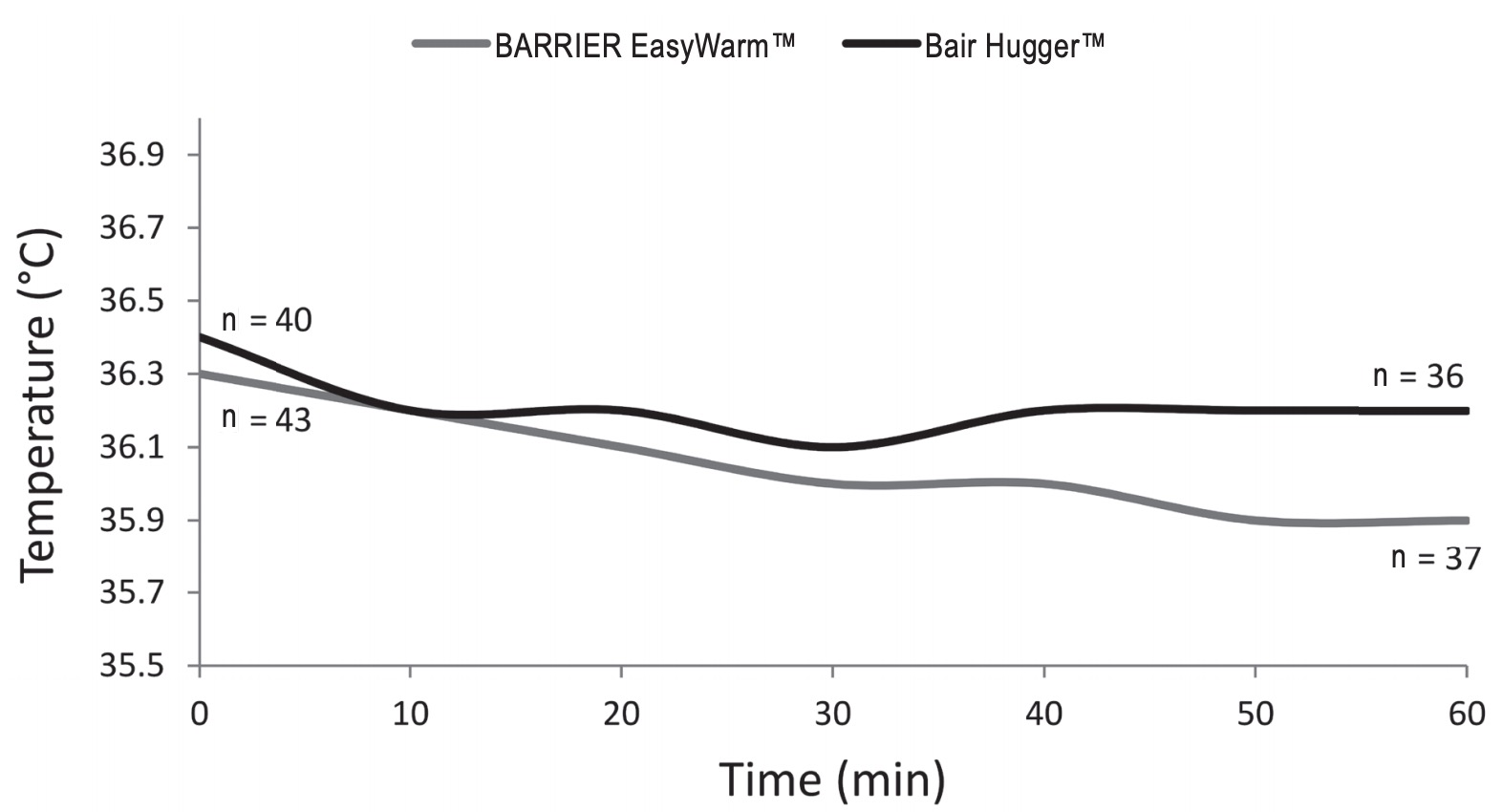
Table 2 shows outcome measures. For the primary outcome, the mean lowest measured core temperatures were respectively 35.9°C (± 0.6) for the self-heating blanket (SHB) group and 36.1°C (± 0.5) for the forced-air blanket (FAB) group. A secondary non-inferiority test (TOST) showed non-inferiority of the SHB in relation to the predetermined delta of 0.5°C. In relation to the zero-point (i.e., no difference between SHB and FAB) the SHB is just inferior by 0.2°C. Fig. 2 shows mean peri-operative temperature in both treatment groups.
Download full-size image
Download full-size image
Mean core temperature before surgery did not differ significantly between the two groups (p = 0.49), nor did mean core temperature after induction of anaesthesia (p = 0.22), at the start of surgery or mean core temperature during surgery (p = 0.68). A significant difference (p = 0.02) in core temperature was found at the end of surgery, 35.9°C (± 0.6) for the SHB group and 36.2°C (± 0.5) for the FAB group. After surgery, at the recovery bay, the SHB group was “colder” compared to the FAB group, 35.8°C (± 0.6) and 36.1°C (± 0.5) respectively (p = 0.04). Measurements below 36.0°C were seen in both groups (SHB = 26/43 [60%], FAB = 15/40 [38%], p = 0.04) and the number of measurements below 36.0°C was comparable; 2.6 ± 2.5 for the SHB group and 2.0 ± 2.7 for the FAB group (p = 0.32). It should be noticed that no postoperative warming was used in either groups because the anaesthesiologists use a temperature of 35°C to decide whether or not a patient is allowed to leave the recovery bay.15 Tympanic temperature measurement showed a mean difference of approximately 0.1°C (range = -1–1.1) compared to the SpotOn™ core thermometer (ICC = 0.814, p < 0.01). Evaluation of patients’ experienced thermal comfort, using a VAS, showed no differences between both groups (p=0.903). The mean VAS was 53.3 (± 15.7) in the SHB group and 52.9 (± 12.3) in the FAB group.
The number of complications was comparable between both groups. In each group, two complications during hospital stay occurred. In the SHB group, one THR treatment was complicated by persistent wound leakage postoperatively. C-reactive protein (CRP) (44) and erythrocyte sedimentation rate (ESR) (31) were elevated. This resulted in surgical debridement and microbial cultures were taken two weeks after the primary surgery. Cultures showed infection (Enterococcus faecalis [E. faecalis] and Staphylococcus lugdunensis [S. lugdunensis]). The patient was treated with antibiotics. The second complication in the SHB group had a history of hemorrhagic stroke. Postoperative clinical signs of aphasia, which was a result of a cerebral infarction, was seen. Acetylsalicylic acid and dypyridamole were started for secondary prophylaxes.
In the FAB group, one THR patient had persistent wound leakage postoperatively. Lab results, showed elevated infection parameters (CRP = 44 mg/L, ESR = 93 mm/hr), which gradually decreased during the postoperative period in several days. The patient was discharged without antibiotics or other intervention. The other complication in the FAB group had also THR. Several days after surgery, the patient was evaluated for tachypnea and hypotension. High infection parameters (CRP = 222 mg/L, ESR = 56 mm/hr) and fever were present; the patient was diagnosed with a urinary bladder infection. Antibiotics were started. The patient improved clinically and was discharged to a temporary rehabilitation clinic. Several days later, the patient presented at the emergency department with sepsis, dehydration, acute renal failure and a metabolic acidosis eventually leading to death.
Discussion
Both intraoperative patient warming methods failed to prevent hypothermia from occurring during the perioperative phase in our study. Measurements below 36.0°C were seen more frequent the SHB group (in 60% of the patients) as opposed to 38% in the FAB group (p = 0.04). The results show that the SHB is less effective compared to the FAB at the end of surgery and postoperatively at the recovery bay. It is important to consider whether the differences between both systems are clinically relevant because apparently, both methods failed to prevent hypothermia. The duration of anaesthesia and surgery was short. Without effective prewarming, redistribution hypothermia will occur and no device will have sufficient time to regain normothermia. This is a possible reason for the high number of patients with measurements below 36.0°C. The prewarming for all patients in the study was the same, using the BARRIER EasyWarm™. The effectiveness of this prewarming method should be evaluated further. The mean core temperature at the holding was 36.5°C (± 0.4) in the SHB group and 36.5°C (± 0.5) in the FAB group; it should be discussed what temperature should be strived for.
The complications, which occurred in both groups, might be related to hypothermia. Hypothermia affects the immune system. Decreased cell-mediated immunity and natural killer (NK)-cell activity, suppression of B lymphocytes and defective function of T lymphocytes is seen due to hypothermia.16,17
There is also an association with suppressed phagocytic activity and reduced bacterial killing. It could be possible that hypothermia contributes to the immune alterations perioperatively and thereby increase the risk of postoperative complications.16,17
Hypothermia, which could be a result of temperature redistribution due to induction of anaesthesia, fluid loss, and reinfusion during surgery, is difficult to manage with passive methods, making active warming necessary. High incidences of postoperative hypothermia are seen in THR and TKR.5,18 Because hypothermia could result in several complications, it should be managed properly.2The Bair Hugger™ (FAB) system has widely been used in studies on peri-operative warming.19 In contrast to the Bair Hugger™, BARRIER EasyWarm™ (SHB) system is quite new. A randomized study showed that the SHB system was superior to passive thermal insulation.20 However, another randomized study reported that a thermal reflective blanket was not able to prevent hypothermia during surgery.19
This finding is consistent with our study. A Cochrane systematic review and meta-analysis comparing forced-air with active resistive heating was unable to show differences in terms of thermal comfort and in terms of postoperative blood loss.19This study has several strengths and limitations. In this single-center randomized controlled non-inferiority trial we were able to randomise 86 of 90 consecutive TKR and THR patients without any loss to follow-up. Core temperature from all patients was measured in a uniform way. The reliability of tympanic temperature measurement is questioned with regard to accuracy compared to core temperature.21 We did not find a relevant difference between either recording methods. One factor that might compromise generalisability is that all THR patients were operated in a supine position via the direct anterior approach while the lateral decubitus position is still more frequently used in hip replacement surgery.
Another possible limitation of our study is that a considerable amount of patients dropped below 36.0°C. Another factor, applicable for both groups, is that the patient could not be fully covered by the SHB nor the FAB due to the sterile field, with a larger uncovered field during THR compared to TKR.
Dasari et al. described the effect of forced-air warming on the laminar flow.9 We did not evaluate the effect of the used blanket on the laminar flow. It would be interesting to evaluate the effect of the blanket and compare it to FAB. The surgeries were performed in different operating theaters, laminar flow was tested and worked properly in all of them but there was no analysis made whether there was a difference between them. Another discussable aspect is the area of the patient that was covered by the blankets; the blankets come in one size and therefore the covered area varies. It could be possible that there is more heating loss in patients who are less covered by the blankets. Unfortunately, this hypothesis is not tested in our study. Also, the blankets were not applied by the same person in all patients, so there could be some variation but the personnel was instructed to apply the blanket in a way that most of the skin was covered.
One of the potential advantages of the self-warming blanket is the possibility to use it continuously; before during and after surgery, there is a constant active warming of the patient possible without interruption. The Bair Hugger™ was turned on as soon as the sterile draping procedure was finished, the time during which the patient was moved from the bed to the operating table until finishing the sterile draping procedure of the surgical site no active warming was used for the patient.
An advantage for the surgical team of a self-warming blanket over a FAB is the comfort of the operating team during surgery. A forced-air device has continuous flow of warm air affecting the surrounding air; if it is close to the operating staff, it could feel quite “hot.” The warmth a self-warming blanket generates remains close to the patient, possibly affecting the surrounding air less and thereby operating staffs’ comfort. Another factor affecting the staff’s comfort is the amount of noise in the OR; it goes without saying that a blanket is quiet while forced-air devices contribute to noise pollution in the OR.19
In this study, both warming blankets did not prevent hypothermia from occurring in both the self-warming blanket group as well as in the FABgroup. However, hypothermia occurred less frequent in the FAB group.
A statistical significant difference between both groups was found in core temperature at the end of TKR or THR surgery. Whether the difference of 0.2°C is clinically relevant, remains to be evaluated; it was nevertheless less than the hypothesised difference of 0.5°C for our non-inferiority study. For that matter, the self-warming blanket was non-inferior to the FAB. But since many patients in both groups showed hypothermia, this should be addressed better.
At the end of surgery and at the recovery room the BARRIER EasyWarm™ group had significant, although little, lower core temperatures; whether such a small difference is clinically relevant, remains to be discussed.
We should ask ourselves the question if it is more important to keep the patient normothermic by using the Bair Hugger™ with slightly better results, but with the theoretical risk of an infection due to interruption of the laminar flow and thereby affecting the sterile field. Perhaps it would be better to optimise the SHB protocol, without interrupting the air flow and thereby reducing the risk of infection. However, hypothermia is also associated with infections. Both aspects, managing normothermia and avoiding interruption of laminar air flow, should be optimized to reduce the risk of infection and therefore further research is needed.
References
| 1 |
Sessler DI.
Temperature monitoring and perioperative thermoregulation.
Anesthesiology 2008;109:318–338.
|
| 2 |
Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A.
Mild hyporthermia increases blood loss and transfusion requirements during total hip arthroplasty.
Lancet 1996;347:289–292.
|
| 3 |
Koëter M, Leijtens B, Koëter S.
Effect of thermal reflective blanket placement on hypothermia in primary unilateral total hip or knee arthroplasty.
J Perianesth Nurs 2013;28: 347–352.
|
| 4 |
Kurz A, Sessler DI, Lenhardt R; the Study of Wound Infection and Temperature Group.
Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization.
N Eng J Med 1996;334:1209–1216.
|
| 5 |
Reina N, Fennema P, Hourlier H.
The impact of mild peri-operative hypothermia on the effectiveness of tranexamic acid in total hip arthroplasty.
Int Orthop 2017;41:55–60.
|
| 6 |
Fanelli A, Danelli G, Ghisi D, Ortu A, Moschini E, Fanelli G.
The efficacy of a resistive heating under-patient blanket versus a forced-air warming system: a randomized controlled trial.
Anesth Analg 2009;108:199–201.
|
| 7 |
Tjoakarfa C, David V, Ko A, Hau R.
Reflective blankets are as effective as forced air warmers in maintaining patient normothermia during hip and knee arthroplasty surgery.
J Arthroplasty 2017;32:624–627.
|
| 8 |
Legg AJ, Hamer AJ.
Forced-air patient warming blankets disrupt unidirectional airflow.
Bone Joint J 2013;95-B:407–410.
|
| 9 |
Dasari KB, Albrecht M, Harper M.
Effect of forcedair warming on the performance of operating theatre laminar flow ventilation.
Anaesthesia 2012;67:244–249.
|
| 10 |
Kellam MD, Dieckmann LS, Austin PN.
Forced-air warming devices and the risk of surgical site infections.
AORN J 2013;98:354–366.
|
| 11 |
Mölnlycke Health Care AB.
BARRIER EasyWarm.
https://www.molnlycke.co.uk/products-solutions/barrier-easywarm. Accessed June 11, 2018.
|
| 12 |
Eshraghi Y, Nasr V, Parra-Sanchez I, et al.
An Evaluation of a Zero-Heat-Flux Cutaneous Thermometer in Cardiac Surgical Patients.
Anesth Analg 2014;119:543–549.
|
| 13 |
Brandt S, Oguz R, Hüttner H, et al.
Resistive-polymer versus forced-air warming: comparable efficiacy in orthopedic patients.
Anesth Analg 2010;110:834–838.
|
| 14 |
Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group.
Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement.
JAMA 2006;295:1152–1160.
|
| 15 |
Gruber A, Behringer W, Knosp E.
Hypothermia in the operating theatre.
Crit Care 2012;16(Suppl 2):A17.
|
| 16 |
Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H.
Effects of mild perioperative hypothermia on cellular immune responses.
Anesthesiology 1998; 89:1133–1140.
|
| 17 |
Brand JM, Frohn C, Luhm J, Kirchner H, Schmucker P.
Early alterations in the number of circulating lymphocyte subpopulations and enhanced proinflammatory immune response during opioid-based general anesthesia.
Shock 2003;20:213–217.
|
| 18 |
Leijtens B, Koëter M, Kremers K, Koëter S.
High incidence of postoperative hypothermia in total knee and total hip arthroplasty.
J Arthroplasty 2013;28:895–898.
|
| 19 |
Madrid E, Urrutia G, Roqué i Figuis M, et al.
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.
Cochrane Database Syst Rev 2016:4:CD009016.
|
| 20 |
Torossian A, Van Gerven E, Geertsen K, Horn B, Van de Velde M, Raeder J.
Active perioperative patient warming using a self-warming blanket (BARRIER EasyWarm) is superior to passive thermal insulation: a multinational, multicenter, randomized trial.
J Clin Anesth 2016;34:547–554.
|
| 21 |
Hooper VD, Andrews JO.
Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science.
Biol Res Nurs 2006;8:24–34.
|